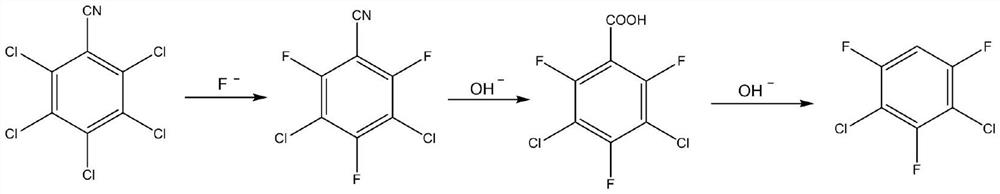
Preparation method of 1, 3-dichloro-2, 4, 6-trifluorobenzene
A technology of trifluorobenzene and trifluorobenzonitrile, which is applied in the preparation of organic compounds, nitriles, and carboxylic acid nitrile, can solve the problems of long time and high reaction temperature, and achieves easy operation, high yield and content, Process saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First step response:
[0025] Take a 2000mL four-neck bottle, connect the stirring, condenser and thermometer casing, add 275.3g of pentachlorobenzonitrile, 680g of DMF, 232g of potassium fluoride and 8.5g of hexamethylguanidine fluoride, stir evenly and raise the temperature to 110°C, and keep it warm for 8h , until the content of difluorotrichlorobenzonitrile ≤ 3%, cooling and filtering. DMF was then concentrated to obtain 185 g of 3,5-dichloro-2,4,6-trifluorobenzonitrile, with a yield of 82%.
[0026] Second step reaction:
[0027] Prepare a total of 540g of 30% sodium hydroxide solution, raise the temperature to 100°C, add 185g of 3,5-dichloro-2,4,6-trifluorobenzonitrile dropwise while stirring, and keep it warm for 6 hours until the raw materials are complete The response is over. The temperature was raised to 130° C., and the decarboxylation reaction was continued for 6 hours until the content of 3,5-dichloro-2,4,6-trifluorobenzoic acid was ≤0.5%, and the react...
Embodiment 2
[0029] First step response:
[0030] Take a 2000mL four-neck bottle, connect the stirring, condenser and thermometer sleeve, add 275.3g of pentachlorobenzonitrile, 720g of DMF, 261g of potassium fluoride and 15g of hexaethylguanidine chloride, stir evenly and raise the temperature to 130°C, keep the temperature for 5h, Until the content of difluorotrichlorobenzonitrile is ≤3%, cool down and filter. DMF was then concentrated to obtain 189.8 g of 3,5-dichloro-2,4,6-trifluorobenzonitrile, with a yield of 84%. Second step reaction:
[0031] Prepare a total of 600g of 20% sodium hydroxide solution, raise the temperature to 105°C, add 189.8g of 3,5-dichloro-2,4,6-trifluorobenzonitrile dropwise while stirring, and keep warm for 7.5h after the dropwise addition, until The raw materials are completely reacted. The temperature was raised to 140° C., and the decarboxylation reaction was continued for 5 hours until the content of 3,5-dichloro-2,4,6-trifluorobenzoic acid was ≤0.5%, and ...
Embodiment 3
[0033] First step response:
[0034] Take a 2000mL four-necked bottle, connect the stirring, condenser and thermometer casing, add 330g of pentachlorobenzonitrile, 924g of DMF and 243.6g of potassium fluoride, stir evenly and raise the temperature to 150°C, keep the temperature for 10h, until difluorotrichlorobenzonitrile Content ≤ 3%, cool down and filter. DMF was then concentrated to obtain 184.4 g of 3,5-dichloro-2,4,6-trifluorobenzonitrile, with a yield of 68%.
[0035] Second step reaction:
[0036] Prepare a total of 540g of 30% sodium hydroxide solution, raise the temperature to 100°C, add 184.4g of 3,5-dichloro-2,4,6-trifluorobenzonitrile dropwise while stirring, and keep warm for 7 hours after the dropwise addition until the raw material Completely reacted. The temperature was raised to 140° C., and the decarboxylation reaction was continued for 5.5 hours until the content of 3,5-dichloro-2,4,6-trifluorobenzoic acid was ≤0.5%, and the reaction was completed. Cool ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com