Cancer-causing mouse model as well as establishment method and application thereof
A mouse model and technology for establishing a method, applied in the field of animal models, can solve problems such as genetic instability, unfriendliness, and loss of foreign genes in animal models, and achieve the goal of avoiding host gene inactivation, overcoming technical difficulties, and improving expression levels Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A carcinogenic mouse model constructed by:
[0064] 1. Gene synthesis
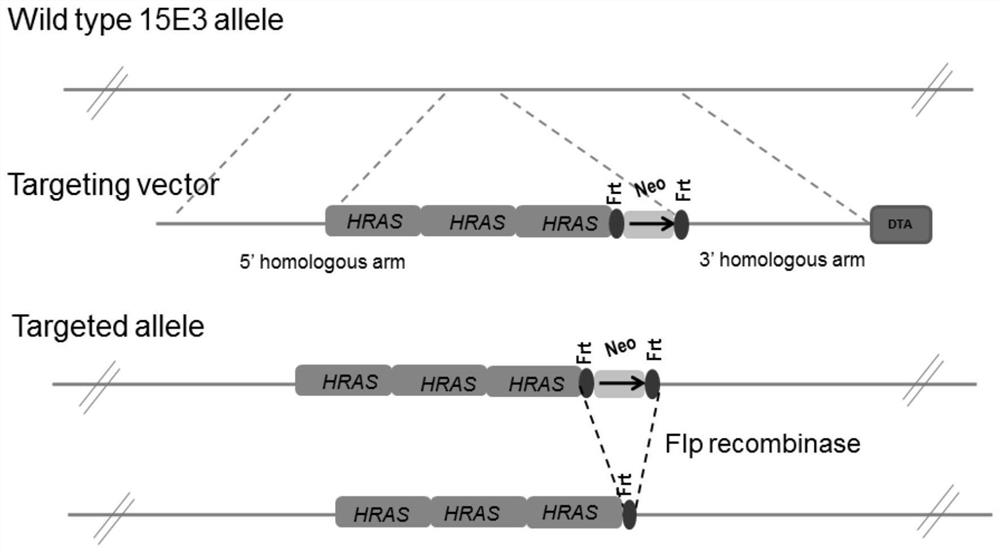
[0065] According to the NCBI database query, the complete sequence of the single-copy human proto-oncogene HRAS was obtained. Through gene synthesis, a single copy of the HRAS sequence was obtained, the sequence of which is shown in SEQ ID NO.1, and inserted into the cloning vector; through in vivo BAC (bacterial artificial chromosome) recombination and screening, a cloning vector containing two copies of the HRAS gene was obtained, Realize two copies in series.
[0066] After direction identification, clones with the correct head-to-tail tandem direction were obtained; through BAC rescue again, the third copy of the HRAS gene was linked to it, and after direction identification, the cloning vector with the correct connection direction and the connection of 3 fragments was selected to form a clone. The 21kb large fragment of the human proto-oncogene HRAS gene contains three copies, and retains the...
Embodiment 2
[0124] In this example, the mouse model obtained above is verified.
[0125] 1. Carcinogenicity verification experiment design
[0126] The test protocol was designed with reference to the technical guidelines on the necessity of drug carcinogenicity tests issued by NMPA, and the S1A and S1B guidelines issued by ICH (REF1-4), and the test items were implemented in accordance with the content of drug carcinogenicity evaluation under GLP conditions.
[0127] The test consisted of a vehicle control group and a positive test group respectively, the dosage was 75 mg / kg, the administration method was a single intraperitoneal injection, and the positive carcinogen was methylnitrosourea (MNU).
[0128] The positive test group was divided into two groups: (1) transgenic mouse MNU group (KI.C57-HRAS, or KI.C57-rasV2.0), a total of 50 mice, 25 male and female; (2) wild type Mouse MNU group (C57BL / 6), a total of 50 mice, 25 male and 25 male;
[0129] Vehicle control group: (1) Transgeni...
Embodiment 3
[0158] Carcinogenicity experiments were performed on the mouse model prepared in Example 1 to verify.
[0159] 1. Verification example 1
[0160] Select KI.C57-rasV2.0 model mice, half male and half male, and divide them into 2 groups by simple randomization according to sex and body weight. Group 1 was a 0.5% CMC-Na negative control group, with 25 animals per sex. Group 2 is the MNU positive control group (N-methylnitrosourea, the dose is 80 mg / kg), with 15 animals per sex. The first group was administered orally once a day for 26 weeks. On the first day, group 2 was intraperitoneally injected with methylnitrosourea (MNU), once.
[0161] The experimental results showed that: in the 0.5% CMC-Na negative control group, all male animals survived to the planned dissection day, and no unplanned death occurred; 1 female animal died on D79, and the rest survived to the planned dissection day. In the positive control MNU group, the first death time of male animals was D53, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com