Secondary T-cell-dependent antibody responses to detect immunosuppressive methods for exogenous compounds
An antibody reaction, exogenous technology, applied in the screening of compounds, detection of programmed cell death, biological testing, etc., can solve specific T cell-dependent antigen insensitivity, large amount of tested exogenous compounds, difficult early stage Drug screening and other issues, to achieve the effect of short immune response time, small inter-individual differences, and strong response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
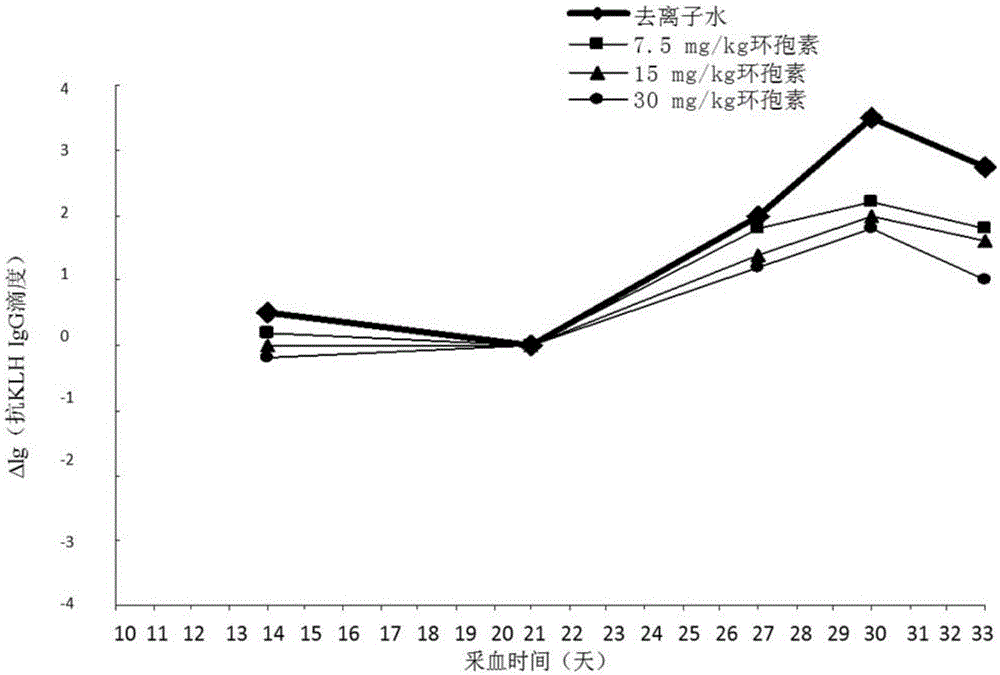
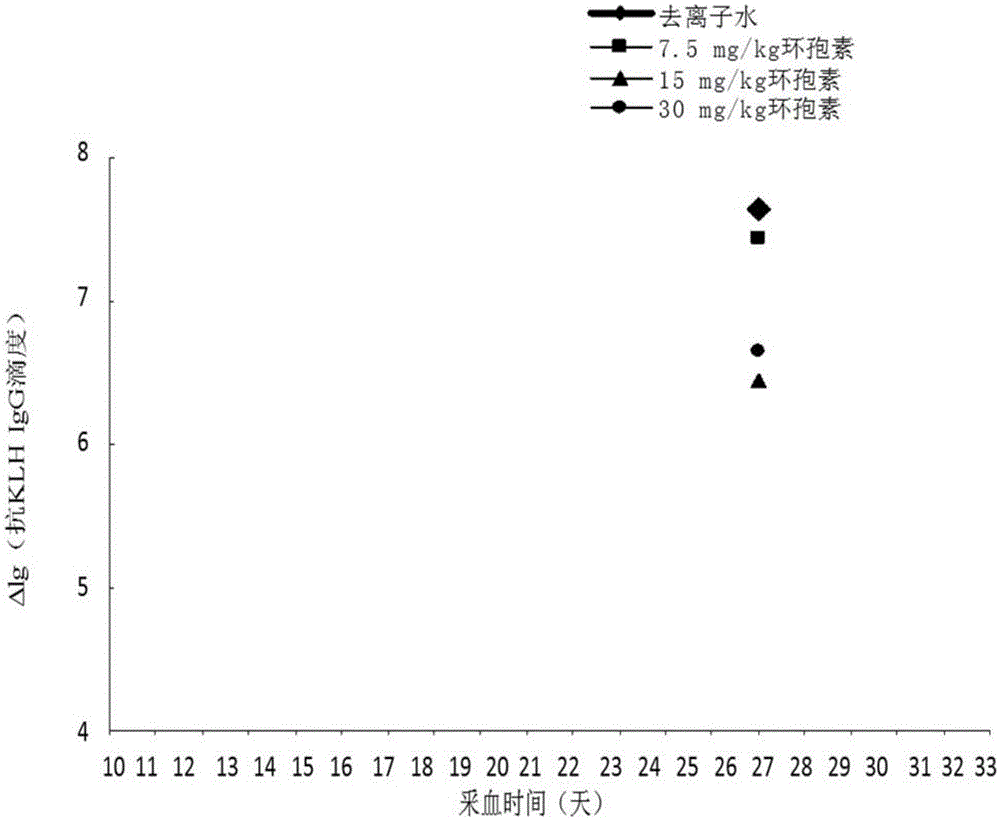
[0032] Experimental method: Select 20 SD female rats with good growth and the same size, and inject 300 μg / kg KLH into the tail vein; 21 days after the first injection of KLH, inject 300 μg / kg KLH intravenously again to stimulate secondary immunity Response, the rats to be tested were equally divided into 4 groups:
[0033] At the same time as the second injection of KLH, deionized water, 7.5, 15, and 30 mg / kg cyclosporine were given orally once respectively; peripheral blood was collected, serum was separated, and anti-KLHIgG and peripheral blood white blood cell count were detected by ELISA method 10 days after the last oral administration of deionized water and different concentrations of cyclosporine, the organs were dissected for histopathological examination.
[0034] Experimental results: peripheral blood leukocyte counts showed that secondary immune 15, 30mg / kg cyclosporine group appeared immune function suppression as shown in Table 1;
[0035] The effects of differe...
Embodiment 2
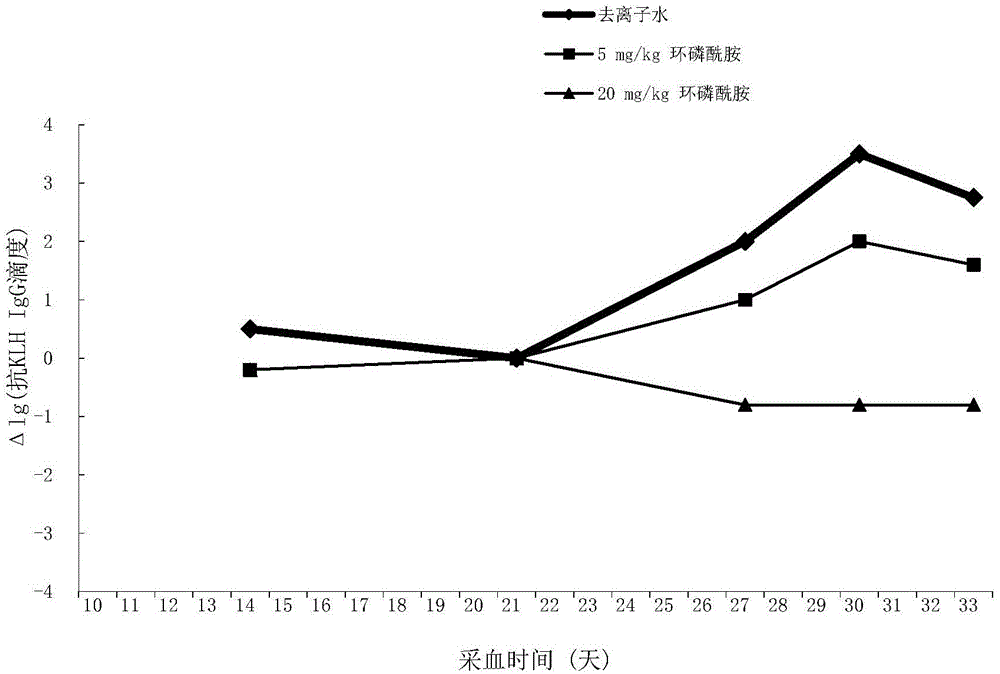
[0037] Experimental method: Select 15 SD female rats with good growth and the same size, and inject 300 μg / kg KLH into the tail vein; 21 days after the first injection of KLH, inject 300 μg / kg KLH intravenously again to stimulate secondary immunity Response, the rats to be tested were divided into 3 groups on average:
[0038] At the same time as the second injection of KLH, deionized water and cyclophosphamide at 5 and 20 mg / kg were given orally once respectively; peripheral blood was collected, serum was separated, and anti-KLHIgG and peripheral blood white blood cell count were detected by ELISA method. On the 10th day after oral administration of deionized water and cyclophosphamide of different concentrations, the organs were dissected for histopathological examination.
[0039] Experimental results: the peripheral blood leukocyte count shows that the secondary immune function of 20mg / kg cyclophosphamide is suppressed as shown in Table 1; it can be seen that after 3 days ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com