Method for determining titer of reovirus type 3 through tissue half infection method staining
A reovirus, half-infection technology, applied in the field of biomedicine, can solve problems that have not been discovered yet, and achieve the effect of reducing errors, strong repeatability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Tissue half infection method staining and determination of Reo-3 virus titer specific implementation method
[0033] First, after the Reo-3 virus was thawed, after ultrasonication and filtration, a 10-fold serial dilution was made according to the actual situation. The virus dilution was EMEM medium with HEPES as a buffer, and the concentration of HEPES was 0.25mol / L;
[0034] Next, add MEM medium in the cell culture 96-well plate, the fetal calf serum content of the MEM medium is 8%, inoculate LLC-MK2 cells in the cell culture 96-well plate, inoculate about 4× in each cell well 104 cells at 37°C, 5% CO 2 Carry out cell culture under culture conditions, observe the cells on the second day, and proceed to subsequent operations when the cell confluence is 70-100%, and the number of inoculated LLC-MK2 cells can be adjusted within a reasonable range to make the cell confluence reach within two days 70-100% can be followed up;
[0035] Again, use the prepared Reo...
Embodiment 3
[0040] Example 3 The same operator carried out the comparison of Reo-3 virus infection LLC-MK2 cell experiment at different times
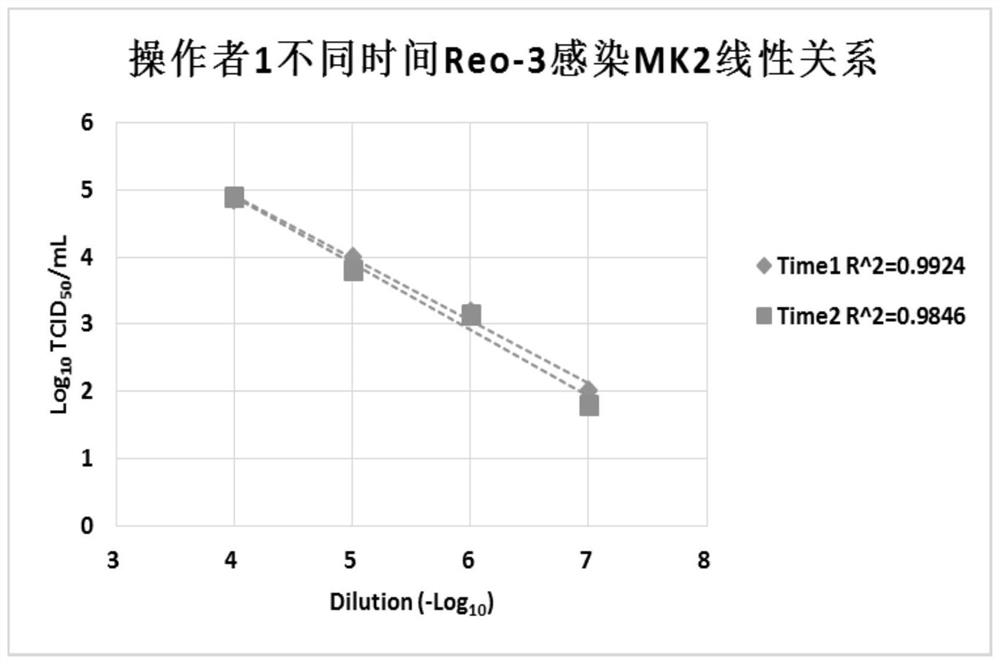
[0041] According to the operation process of Example 1, the experimental operator Operator1 carried out the titer determination experiments Run1 and Run3 of the Reo-3 virus on the first day and the second day respectively. Repeatedly, after serial dilution of the sample, do 8 multiple hole experiments for each dilution, exclude samples whose virus concentration is lower than the detection limit, and select four samples for result analysis, the results are as follows: image 3 shown.
[0042] Depend on image 3 It can be seen that at two different time points, Reo-3 virus infected LLC-MK2 cells, and at different dilution factors, all showed a good linear relationship, and the results were stable and reproducible.
Embodiment 4
[0043] Comparison of different experimental operators in embodiment four
[0044] According to the operation process of Example 1, two experimental operators, Operator 1 and Operator 2, independently carried out experiments at the same time, Run1 and Run2, each Run contained 6 samples of experiments, each sample was repeated three times, and the samples were serially diluted , do 8 duplicate hole experiments for each dilution, exclude the samples whose virus concentration is lower than the detection limit, select four of them for result analysis, the results are as follows Figure 4 shown.
[0045] Depend on Figure 4 It can be seen that there is no significant difference between Operator 1 and Operator 2, and both operators show a good linear relationship under different dilution factors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com