Exosome delivery carrier as well as preparation method and application thereof
A delivery carrier and exosome technology, applied in the medical field, can solve the problems of GBM prone to drug resistance, impossible complete surgical resection, poor curative effect, etc., and achieve good blood-brain barrier penetration ability and low immunogenicity , the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The preparation and application of the drugs for treating neurodegenerative diseases include but are not limited to enhancing immunity, slowing down aging and memory loss. In one embodiment, the above-mentioned exosome delivery carrier or pharmaceutical composition can be prepared as a neuron protection agent and a neuron repair agent.
[0079] It should be noted that the above listed are only several possible diseases listed by the inventor. In other embodiments, as long as the effects of relieving and curing neurodegenerative diseases can be achieved, they are all within the scope of protection of the present invention, and not It is not limited to the types of several diseases listed above.
[0080] It should be noted that the preparation and application of the above-mentioned tumor treatment drugs include not limited to the effects of tumor focus elimination, tumor size reduction, anti-tumor, tumor drug resistance treatment, etc., and are not limited to the types of...
Embodiment 1
[0091] This example provides a method for preparing an exosome delivery carrier.
[0092] (1) Exosome isolation
[0093] The separation protocol was modified from the standard protocol. Bone marrow mesenchymal stem cells at 75cm 2 Cultured in a cell culture flask, DMEM-F12 medium was added with 10% exosome-free FBS (120,000×g, centrifuged at 4°C for 12 hours, and the precipitate was discarded) plus 1% streptomycin penicillin solution, after the cells were confluent The cell fusion rate was 90% without changing the medium for 72 hours. At this time, the cell culture medium was collected in a 50ml test tube, centrifuged at 2000×g for 20 minutes, and then centrifuged at 15000×g for 30 minutes at 4°C. The supernatant was then collected and filtered through a 0.22 μm sterile filter to ensure that large particles and macromolecules were filtered out. Finally, the medium was centrifuged at 120,000×g for 70 minutes at 4°C. The supernatant was discarded, and the exosome pellet pell...
experiment example 1
[0102] In this experimental example, the particle size of the exosome delivery carrier prepared in Example 1 was detected, observed under an electron microscope, and the efficiency of loading siRNA and TMZ on the exosome was detected.
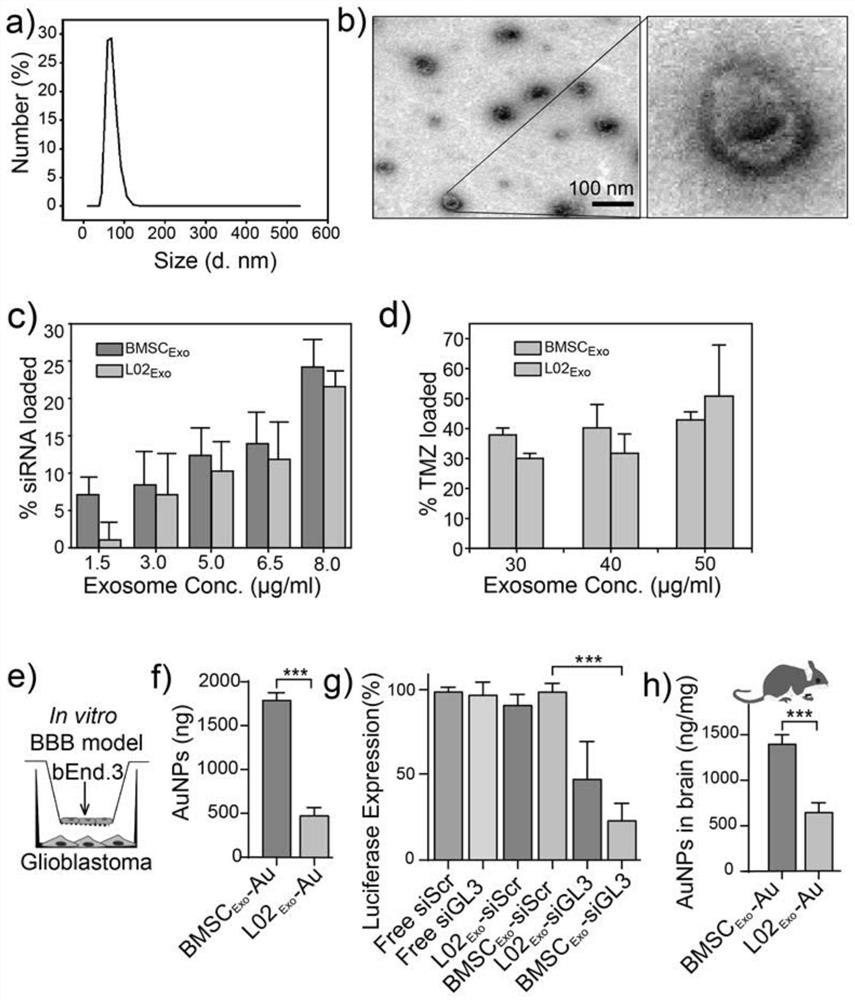
[0103] Result reference figure 1 as shown, figure 1 The a picture in the figure is the size distribution of BMSC-derived exosomes (BMSCExo), the b picture is the transmission electron microscope image, the c picture is the loading percentage of siRNA in BMSCExo and LO2Exo (the abscissa is the exosome concentration), and the d picture is The loading percentage of TMZ in BMSCExo and LO2Exo (abscissa is exosome concentration). Panel e is used for panels f-g, bEnd.3 Transwell assay for in vitro BBB model of endothelial cells The BBB was mimicked in vitro, and penetration efficiency was assessed in GBM cells in the lower chamber. Panel f shows the total penetration (size <10 nm) of AuNPs loaded in BMSCExo and LO2Exo in the transwell model. Figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com