Application of compound P57 or analogs thereof to body temperature reduction and neuroprotection
A technology of compounds and analogs, applied in the fields of steroids, neurological diseases, chemical instruments and methods, etc., which can solve the problems of limited patients and limited time window, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
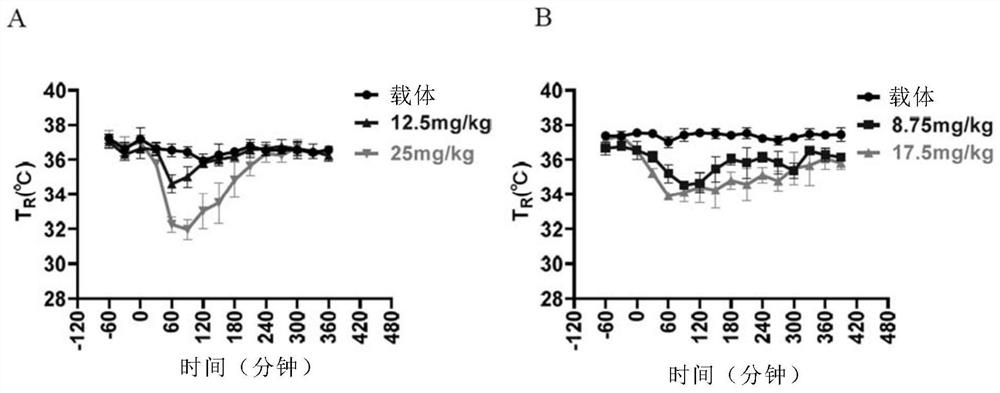
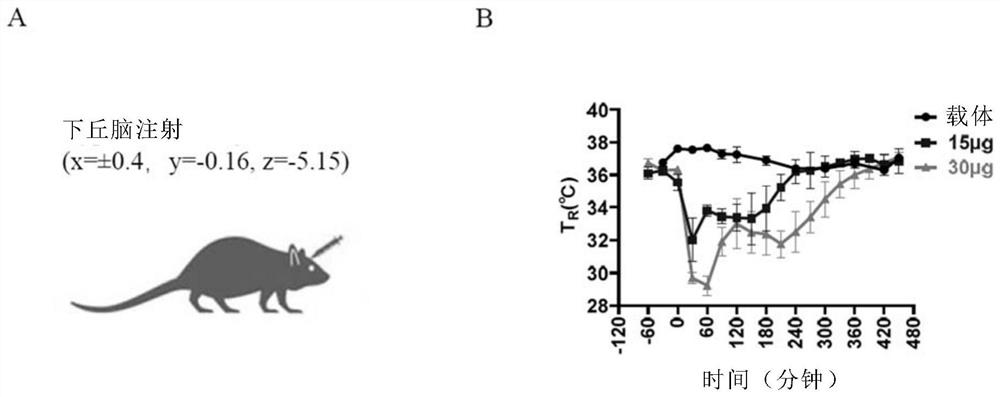
[0052] Embodiment 1, P57 induces hypothermia in mice and rats
[0053] 1.1 Materials
[0054] 1.1.1 C57BL / 6J 6-8 week old male mice
[0055] 1.1.2 Wistar male rats aged 6-8 weeks
[0056] 1.1.3 Dimethylsulfoxide (DMSO)
[0057] 1.1.4 Castor oil
[0058] 1.1.5 Phosphate buffer solution (PBS)
[0059] 1.1.6 75% ethanol solution
[0060] 1.1.7 Lincomycin lidocaine gel
[0061] 1.1.8 Povidone-iodine solution
[0062] 1.1.9 Double tube micro-dosing system for mice
[0063] 1.1.10 1mL disposable sterile syringe
[0064] 1.1.11 5μL Microsyringe
[0065] 1.1.12 1.5mL EP tube
[0066] 1.1.13 15mL centrifuge tube
[0067] 1.2 Equipment
[0068] 1.2.1 Mouse rectal temperature detector
[0069] 1.2.2 Desktop digital stereotaxic instrument
[0070] 1.2.3 Mouse Adapter
[0071] 1.2.4 Mini Round Head Electric Drill
[0072] 1.3 Solution
[0073] 1.3.1 P57 was dissolved in 1% DMSO + 9% castor oil + 90% PBS solution.
[0074] 1.3.2 Preparation of 5% water and chloral solution...
Embodiment 2
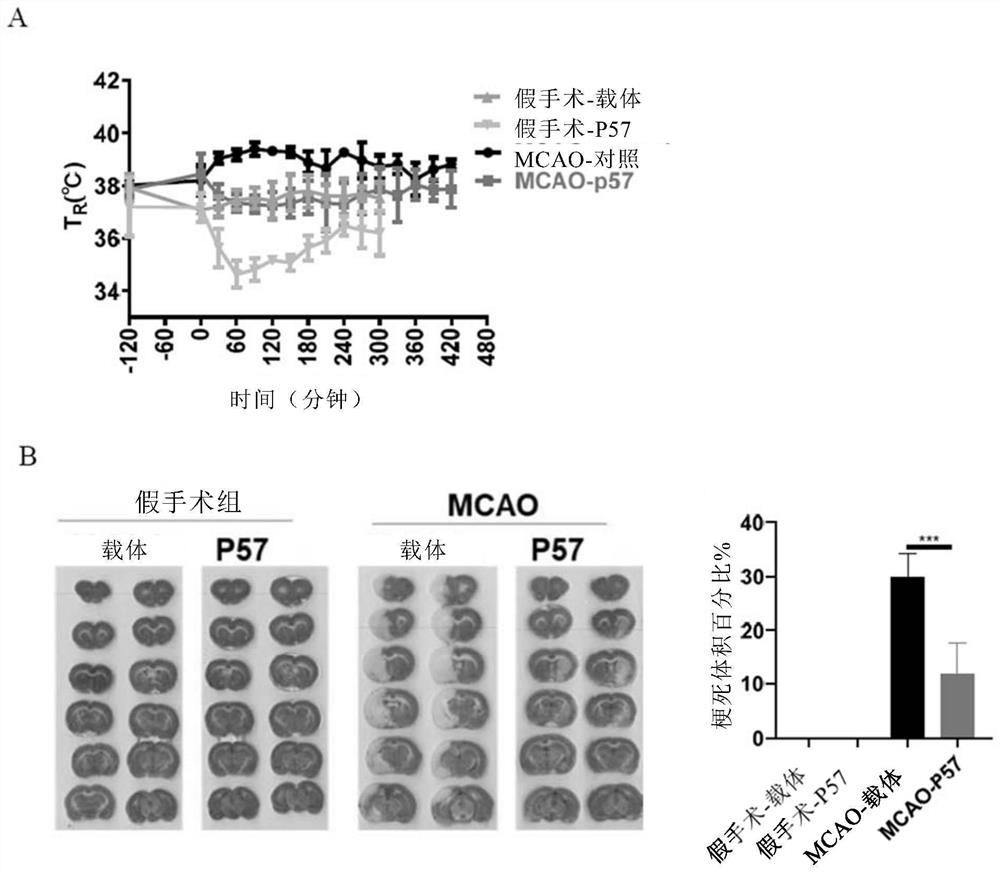
[0086] Example 2 The neuroprotective effect of P57 in the treatment of hypoxic-ischemic diseases
[0087] 2.1 Materials
[0088] 2.1.1 2,3,5-Triphenyltetrazolium Chloride
[0089] 2.1.2 Nylon monofilament suture
[0090] 2.2 Equipment
[0091] 2.2.1 Ventilator
[0092] 2.2.2 Vevo 2100 Micro Ultrasound System
[0093] 2.3 Solution
[0094] 2.3.1 Preparation of 1% 2,3,5-triphenyltetrazolium chloride (2,3,5-triphenyltetrazolium chloride, TTC) solution: Weigh 1 g of TTC and dissolve it in 100 mL of 1×PBS solution.
[0095]2.3.2 Preparation of 4% paraformaldehyde solution: Dissolve 4g of paraformaldehyde in 1×PBS solution, adjust the pH to 7.4, dilute to 100mL, and filter. Protect from light and store at 4°C for later use.
[0096] 2.4 Method
[0097] 2.4.1 Establishment of rat middle cerebral artery occlusion (MCAO) animal model
[0098] Rats of Wistar strain were randomly divided into sham operation blank control group, sham operation drug intervention group, MCAO blank ...
Embodiment 3
[0110] Example 3 Effect of P57 on treating fever
[0111] 3.1 Materials
[0112] 3.1.1 Prostaglandin E2 (PGE2)
[0113] 3.2 Solution
[0114] 3.1.1 PGE2 solution: Dissolve PGE2 to 2M in normal saline.
[0115] 3.3 Method:
[0116] fever model
[0117] The mice implanted with the micro-dosage system two weeks ago and in good health were randomly divided into blank control group, P57 group, PGE2 group, and P57+PGE2 group. Weigh and record the body weight of the mice. One hour before the drug treatment, the body temperature of the mice was recorded by the metabolic cage, P57 (25 mg / kg) was injected intraperitoneally in the P57 group and the P57+PGE2 group, and the other groups were injected with solvent; 1 hour later, the hypothalamus of the PEG2 group and the P57+PGE2 group was injected PGE2 (2μL), and the other groups were injected with solvent.
[0118] 3.4 Results
[0119] In the MCAO model, we found that the body temperature of rats in the control group was higher th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com