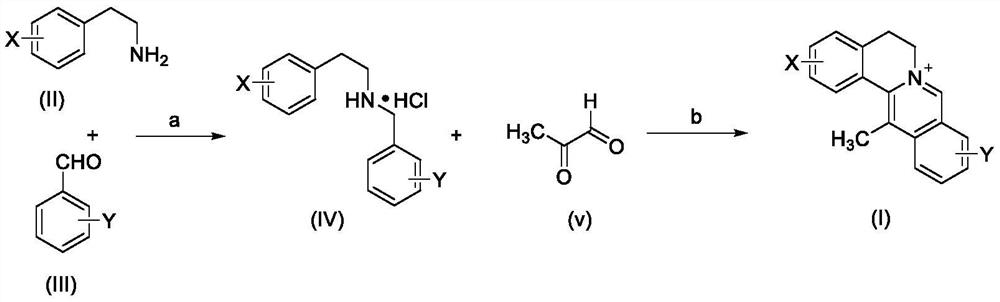
Synthesis method of 13-methyl berberine alkaloid
A technology of methyl berberine and a synthetic method, applied in directions such as organic chemistry, can solve the problems of complicated synthesis steps, complicated operation, high price and the like, and achieve the effects of simplified synthesis steps, easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of 13-methylberberine:
[0046]
[0047] Described step (a) concrete operation:
[0048] 1. After drying the double-necked bottle, add 4AMS powder under the protection of argon, then add 3,4-methylenedioxyphenethylamine and 2,3-dimethoxybenzaldehyde, and use it after drying After the solvent is dispersed evenly, it is placed at room temperature and stirred at 300-400r / min for 12 hours. After the reaction is complete as detected by TLC, the reaction solution is filtered with diatomaceous earth to remove molecular sieves, and the solution is spin-dried to obtain the crude Schiffer's base.
[0049]2. Take the double-neck bottle, after drying, disperse the crude Schiff base with a solvent under the protection of argon, put it in an ice bath to pre-cool, then slowly add sodium borohydride into it, and detect the reaction by TLC. The reaction solution was quenched with saturated ammonium chloride solution under ice bath, the organic solvent was spinned off, an...
Embodiment 2
[0055] Preparation of 13-methylpalmatine base:
[0056]
[0057] The operation in the step (a) is the same as in Example 1, except that arylethylamine is replaced by 3,4-dimethoxyphenethylamine.
[0058] The operation in step (b) is the same as in Example 1 to obtain a yellow solid with a yield of 91%.
[0059] Melting point: 169.3-171.4°C, 1 H NMR(400MHz,DMSO-d6)δ9.91(s,1H),8.21(s,1H),8.19(d,J=9.5Hz,1H),7.39(s,1H),7.18(s,1H) ,4.89–4.82(m,2H),4.11(s,3H),4.09(s,3H),3.89(s,3H),3.86(s,3H),3.14(d,J=5.9Hz,2H), 2.99(s,3H). 13 C NMR(151MHz,DMSO-d6)δ150.63,150.18,147.19,144.03,143.98,136.06,133.08,131.81,129.73,125.91,121.29,120.70,119.12,114.34,111.01,62.04,57.02,56.83,56.18,55.86,26.82 ,17.72
Embodiment 3
[0061] The preparation of 13-methyl coptisine:
[0062]
[0063] The operation in the step (a) is the same as in Example 1, the arylethylamine is 3,4-methylenedioxyphenethylamine, and the aromatic aldehyde is replaced by 2,3-methylenedioxybenzaldehyde.
[0064] The operation in step (b) is the same as in Example 1 to obtain a yellow solid with a yield of 87%.
[0065] Melting point: 231-232.7°C, 1 H NMR (400MHz, DMSO-d 6 )δ9.97(s,1H),8.05(d,J=8.9Hz,1H),7.98(d,J=8.9Hz,1H),7.46(s,1H),7.14(s,1H),6.56( s,2H),6.18(s,2H),4.78(t,J=5.9Hz,2H),3.15–3.06(m,2H),2.91(s,3H). 13 C NMR (101MHz, DMSO-d 6 )δ 148.93, 147.09, 146.36, 144.75, 143.09, 135.42, 133.71, 132.24, 130.74, 120.39, 120.12, 119.51, 110.84, 110.73, 108.16, 104.71, 102.01, 276.19
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com