Preparation method and application of fluorocalciferol CD ring
A technology for calcidol and compounds is applied in the field of preparation of the CD ring of fluorocalcidol, which can solve the problems of expensive raw materials, low safety and high cost, and achieve the effects of high reaction yield, simplified reaction steps and shortened synthesis cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
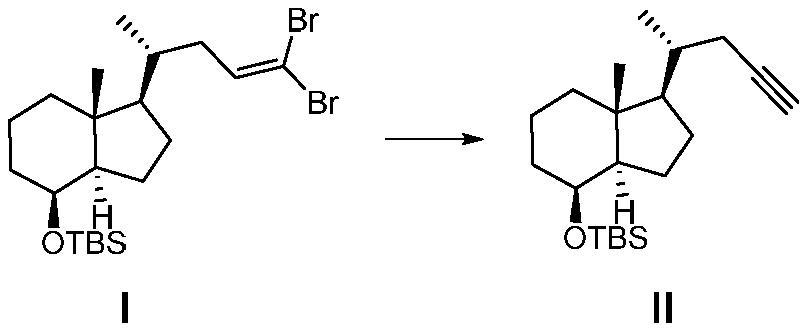
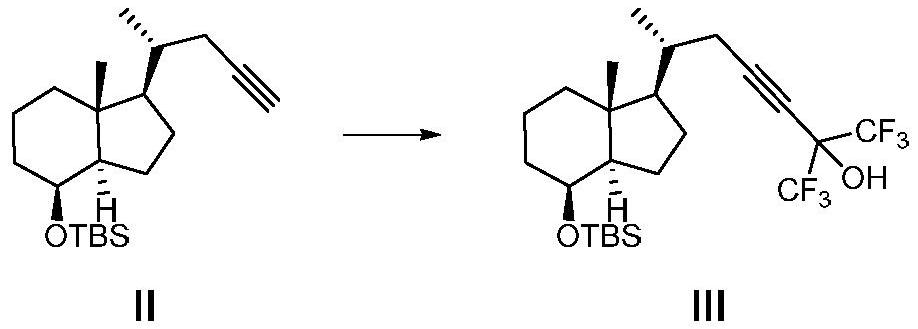
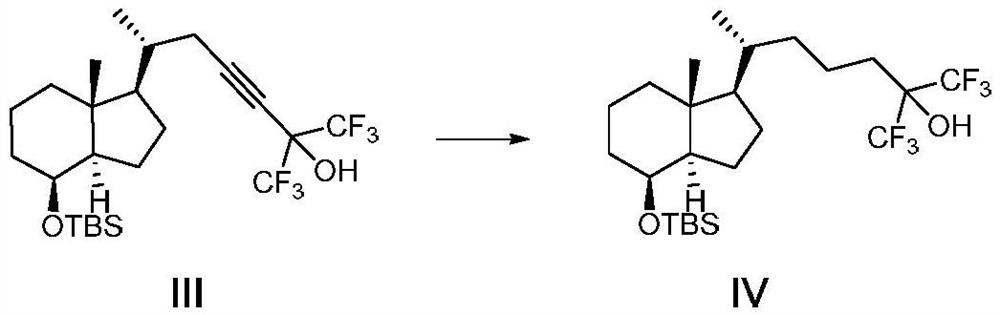
[0047] A method for preparing the CD ring of flucalcidol intermediate, the synthetic route of the method is as follows:
[0048]
[0049] Wherein, R in compound VII is a hydroxyl protecting group, especially a hydroxyl protecting group that can be removed by fluorine-containing inorganic salts, fluorine-containing organic salts, or hydrofluoric acid.
[0050] Compound I was prepared by the method disclosed in the literature Journal of Medicinal Chemistry, 2018, 61(15), 6658-6673, and the rest of the raw materials and reagents were obtained from commercial sources.
[0051] Preparation of compound Ⅱ
[0052] Add compound I (136.0g, 275.1mmol) and tetrahydrofuran (1360.0ml) into a 3000ml four-neck flask, cool the reaction down to -70°C to -80°C, and add n-butyl lithium (439.0ml, 1100.3 mmol), keep the temperature for 1 h, quench the reaction with water, separate the organic phase, dry and concentrate to obtain the crude product, the crude product is 70.2 g by column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com