Method for synthesizing chiral tofacitinib citrate intermediate by enzyme method
A technology for tofacitinib and enzymatic synthesis, which is applied in the fields of botanical equipment and methods, biochemical equipment and methods, enzymes, etc., and can solve the problems of complex operation, poor atom economy, and complicated procedures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment one transaminase thalline freeze-dried powder
[0023] The synthesized transaminase catalyst gene DNA fragment was double-digested with restriction endonuclease NdeI and AvrII at 35-37°C for 6h, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit (SEQ ID NO .1); Then, under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pACYCDuet-B that was digested by NdeI and EcoRI overnight at 25-27°C to obtain a recombinant expression plasmid; transforming the recombinant expression plasmid To Escherichia coli competent cells, the transformation conditions are: 40-45°C, heat shock for 80-90 seconds, screen positive recombinants on a resistance plate containing chloramphenicol, pick a single clone, and culture Recombinant bacteria, after the plasmid is amplified, extract the plasmid, re-transform into competent cells, spread the transformation solution on an L...
Embodiment 2
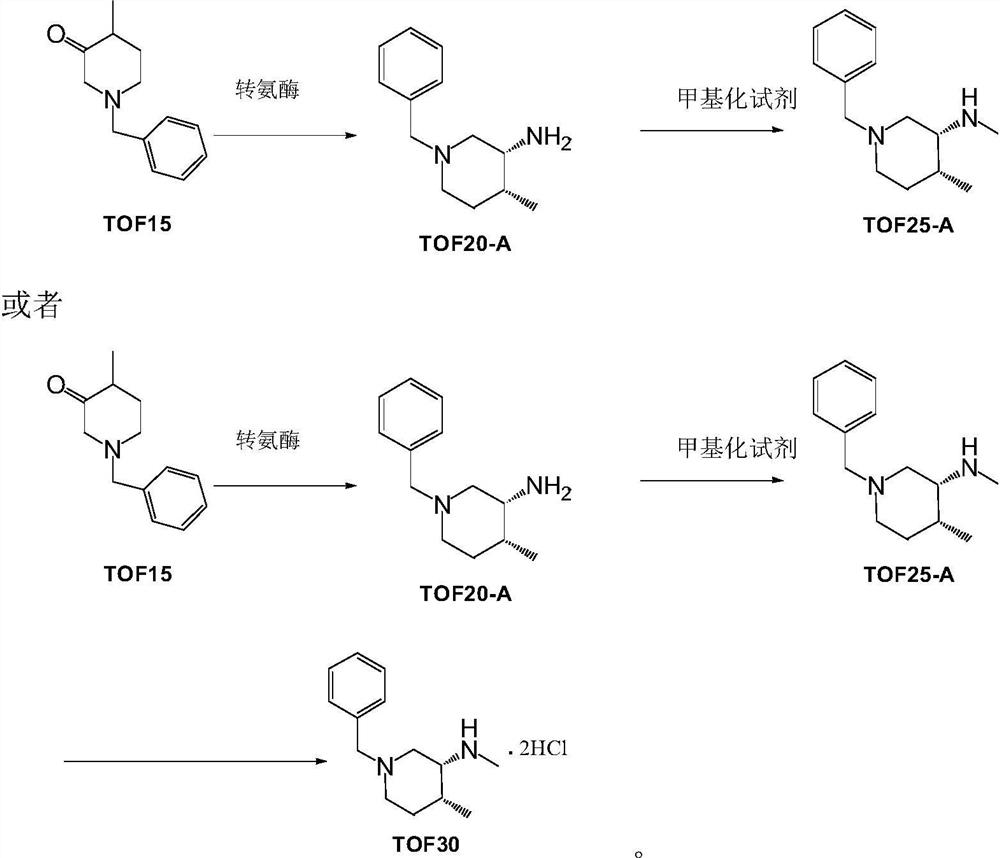
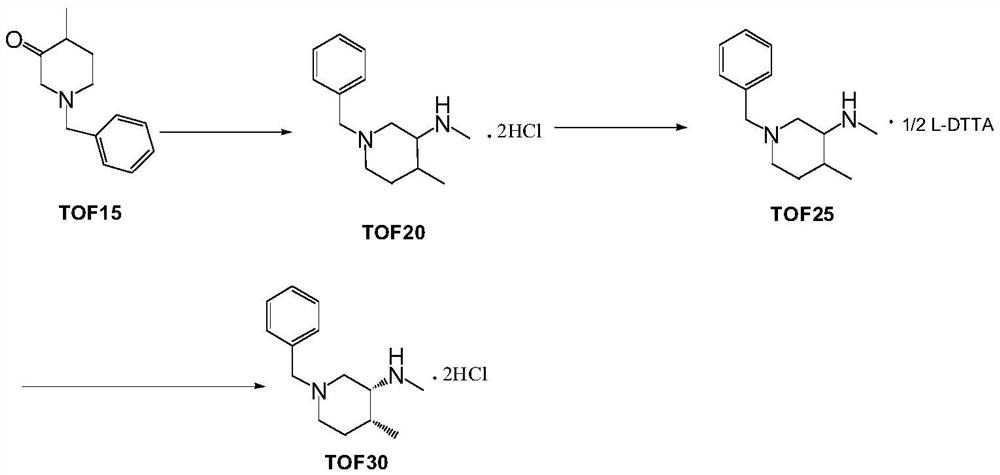
[0024] Preparation of Example 2 TOF20-A
[0025]
[0026] Dissolve 100 g of isopropylamine in 100 ml of water, adjust the pH value to 7.0-8.0 with aqueous hydrochloric acid under cooling in an ice-water bath, and add 10 ml of dimethyl sulfoxide, then dilute to 700 ml with 0.1M Tris-HCl buffer, and pre- Heat to 30°C, then add 100ml of dimethyl sulfoxide solution containing 50g of 4-methyl-1-(phenylmethyl)-3-piperidone (TOF15), and finally add 1g of transaminase lyophilized powder and PLP (Pyridoxal Phosphate) 0.8g, use 20% isopropylamine aqueous solution to control the pH value of 7.0-8.0 for the reaction, convert the temperature at 15-25° C. for more than 12 hours, and monitor the completion of the reaction by TLC. The solid was removed by filtration, the mother liquor was extracted three times with dichloromethane, the combined organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain 45 g of oily TOF20-A, with a yield of about 90%.
Embodiment 3
[0027] Preparation of Example Three (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3-amine (TOF25-A)
[0028]
[0029] Dissolve 10g (about 50mmol) of the oil TOF20-A in Example 1 in 100ml of dichloromethane, add 7g (about 50mmol) of powdered potassium carbonate, cool in an ice-water bath to 0-5°C, and start Iodomethane (7.1 g, about 50 mmol) was added dropwise. After the dropwise addition was completed, the temperature was slowly raised to 15-25° C. and kept for 2 hours. The reaction was monitored by TLC. The solid was removed by filtration, the dichloromethane layer was washed three times with water, the dichloromethane phase was dried over anhydrous sodium sulfate, and concentrated to obtain 10 g of oily TOF25-A, with a yield of about 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com