Antibacterial composition
An antibacterial and composition technology, applied in the direction of antibacterial drugs, drug combination, drug delivery, etc., can solve the problems that it is difficult to treat Acinetobacter infection, and no effective drug has been found, so as to prevent Acinetobacter infection , the effect of reducing the burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0083] [Preparation of sample]
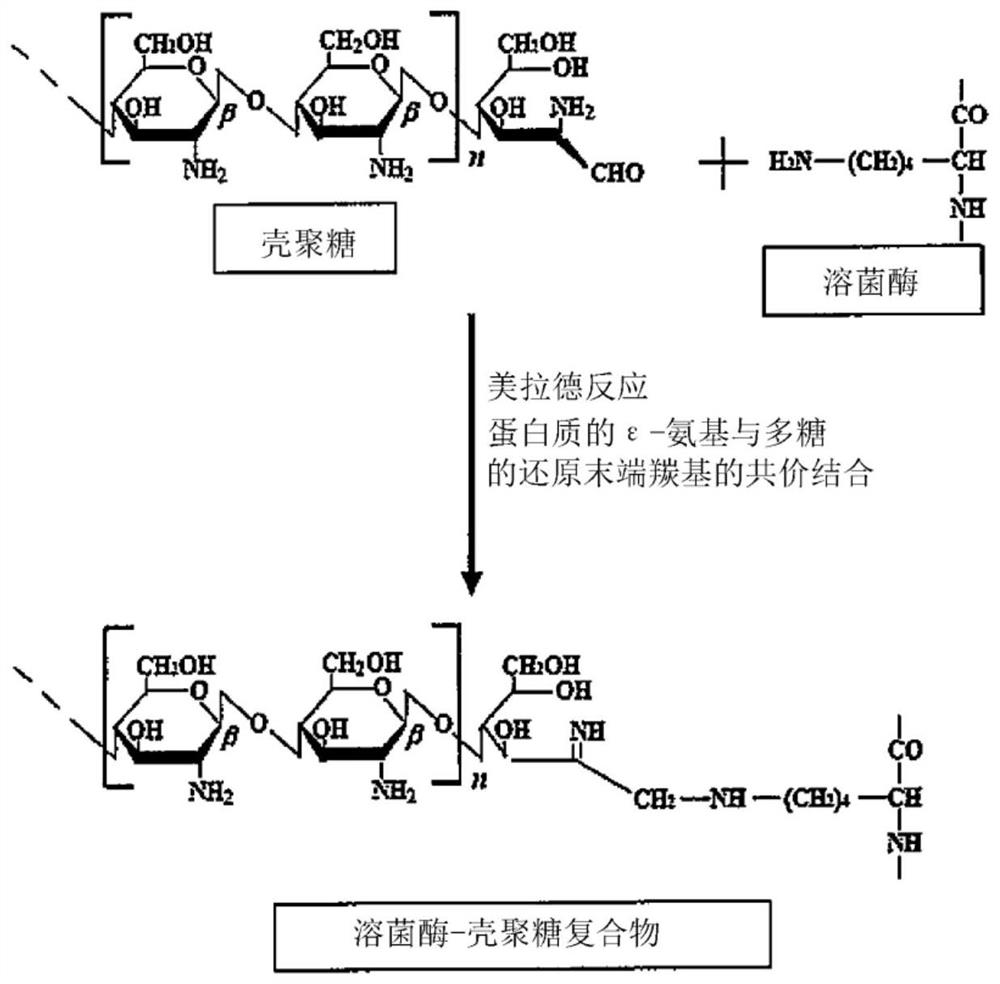
[0084] · Lysozyme-chitosan complex (LYZOX (registered trademark)) solution
[0085] As a solution of a complex (lysozyme-chitosan complex) obtained by binding lysozyme and chitosan, commercially available LYZOX (registered trademark, manufactured by Wako Filter Technology Co., Ltd.) was used. Commercially available LYZOX (registered trademark) is a powder containing chicken-derived lysozyme (about 14000 Da) and water-soluble chitosan (chitooligosaccharide) of about 5000 Da in a mass ratio of 1:1. Specifically, the above-mentioned lysozyme and water-soluble chitosan are mixed and dissolved in water, and after that, they are made into powder by freeze-drying, and further, under the conditions of sufficient temperature, humidity and days for the Maillard reaction to be completely completed, pass The Maillard reaction yielded the lysozyme-chitosan complex (LYZOX (registered trademark)) used in the present invention. The lysozyme-chitosan complex ...
experiment example 1
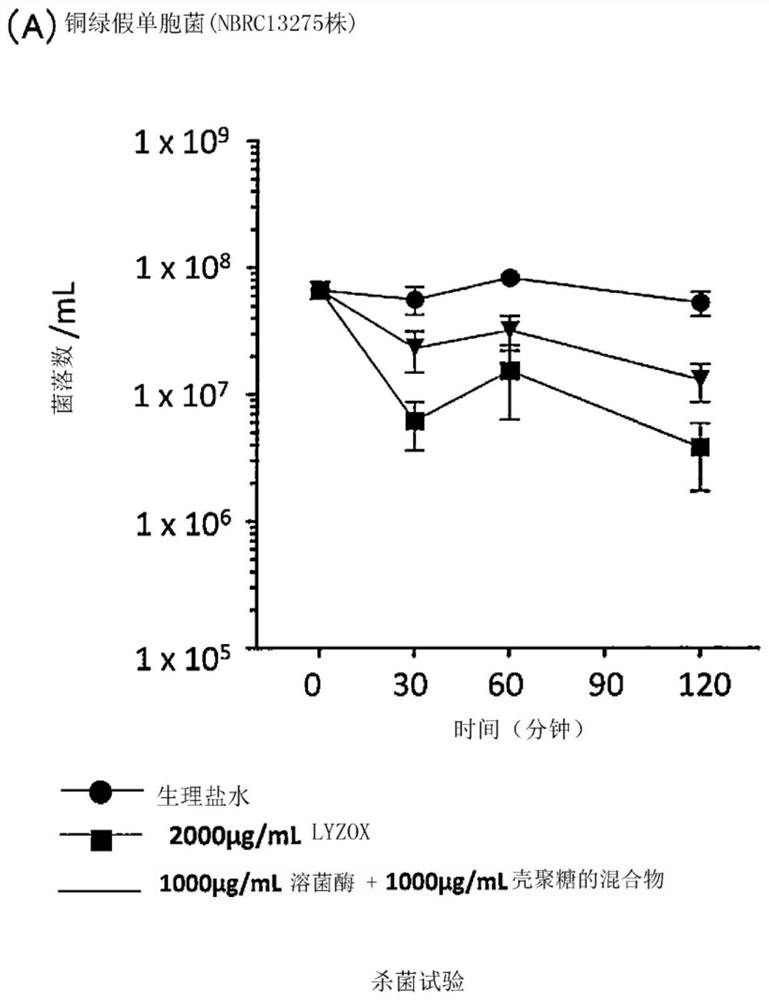
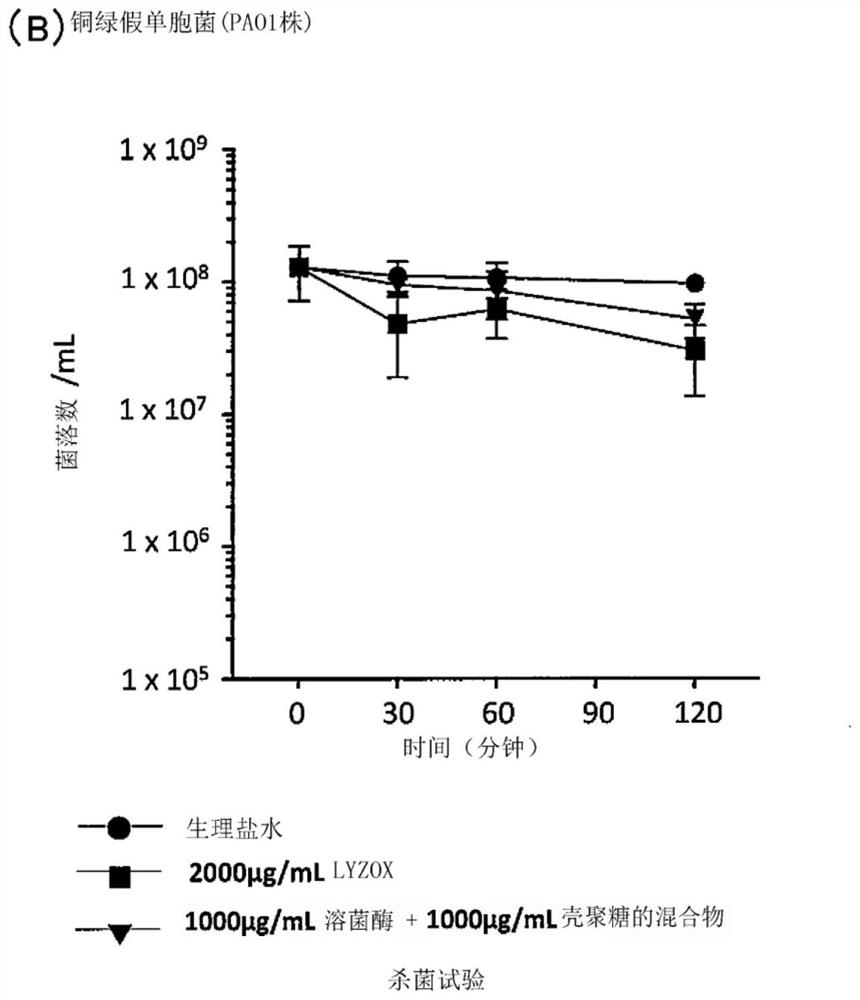
[0099] Experimental example 1 (bactericidal test)
[0100] In comparison with a mixture of lysozyme and chitosan and the like, the bactericidal activity of the lysozyme-chitosan complex (LYZOX (registered trademark)) against Acinetobacter and the like was measured.
[0101] Specifically, for the above-mentioned lysozyme-chitosan complex (LYZOX (registered trademark)) solution (2000 μg / mL, using physiological saline as a solvent), and a solution of a mixture of lysozyme and chitosan (lysozyme monomer solution [1000μg / mL] and chitosan monomer solution [1000μg / mL], use physiological saline as solvent), add 30μL of 1.0M phosphate buffer (pH7.2) in every 1.0mL solution, adjust Each solution was prepared to neutral pH (7.0-7.3). As a control, physiological saline (0.9% NaCl solution) was used. Acinetobacter bacterial suspensions or Pseudomonas aeruginosa bacterial suspensions were prepared as follows: Pseudomonas aeruginosa (NBRC 13275 or PAO1) or Acinetobacter baumannii (JCM 6841...
experiment example 2
[0104] Experimental example 2 (bactericidal test)
[0105] The bactericidal effect of Pseudomonas aeruginosa (NBRC 13275) on each concentration of the lysozyme-chitosan complex (LYZOX (registered trademark)) was measured. Specifically, as the above-mentioned lysozyme-chitosan complex (LYZOX (registered trademark)) solution, physiological saline (0.9% NaCl solution) was used as a solvent, and solutions having different concentrations (200 μg / mL, 2000 μg / mL, and 10000 μg / mL), the experiment was repeated in the same manner as in Experimental Example 1 above, and the bacterial count of Pseudomonas aeruginosa (NBRC 13275) was measured. The results are shown in Figure 2D . like Figure 2D As shown, the lysozyme-chitosan complex exhibited concentration-dependent bactericidal activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com