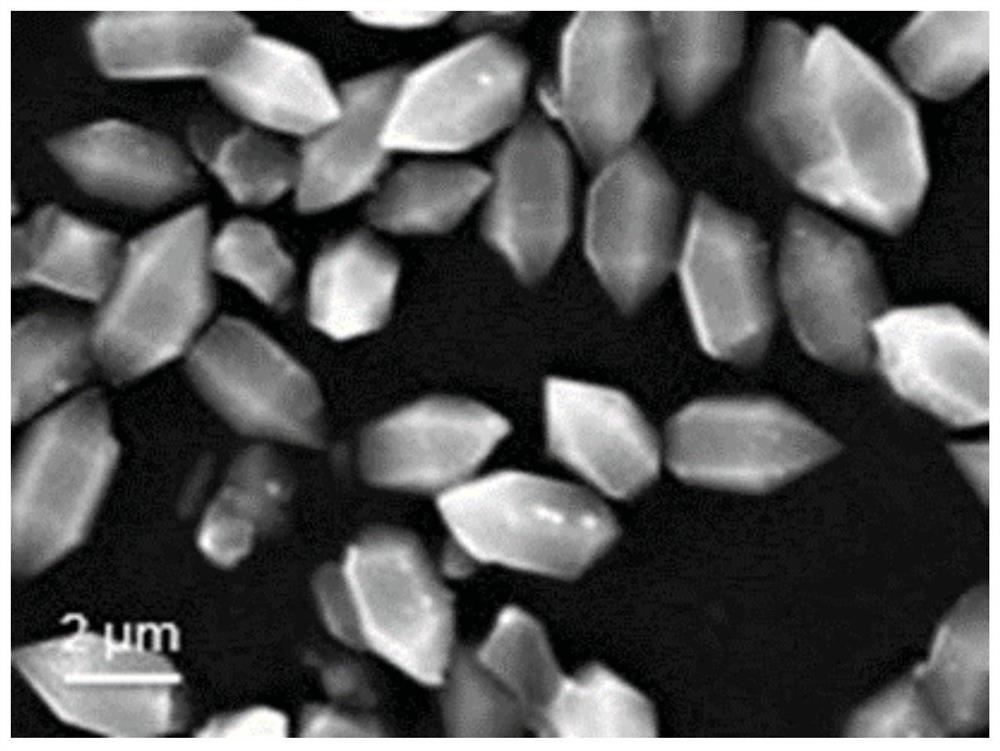
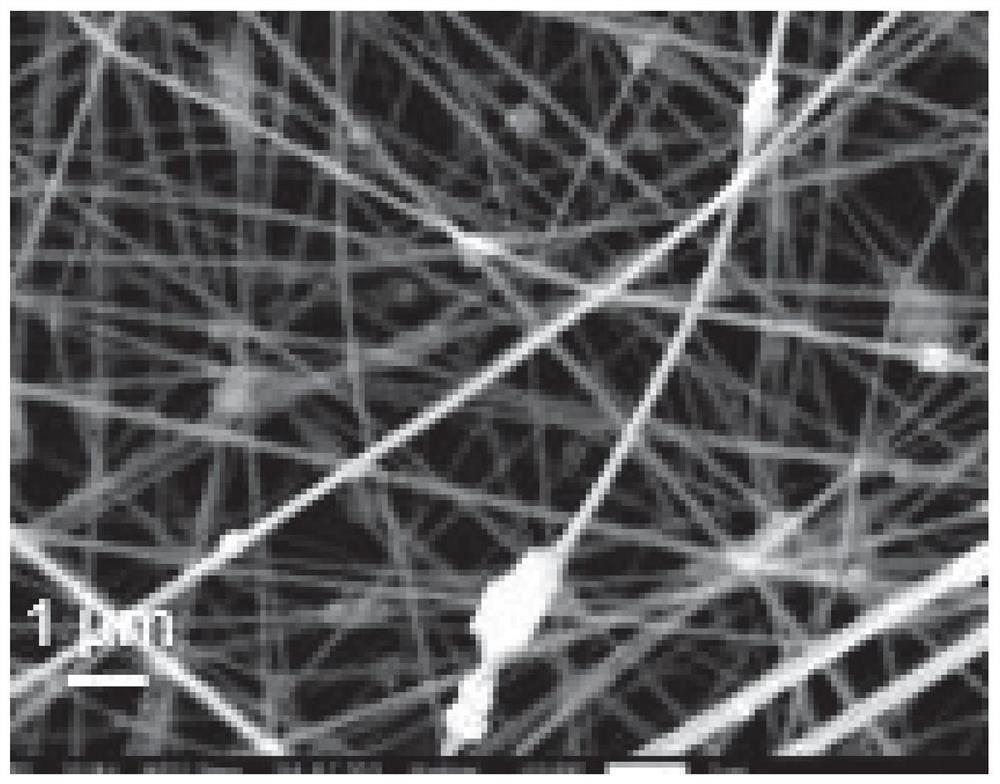
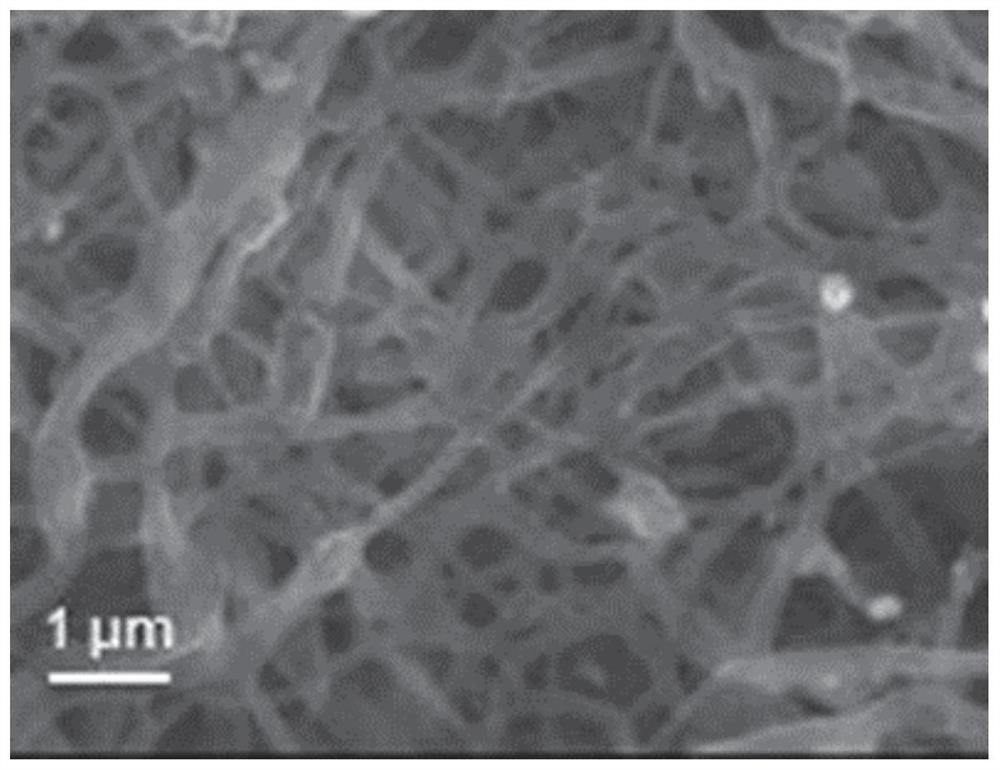
Preparation and application of Fe-N-CNFs catalyst based on Fe-MIL
A fe-n-cnfs and catalyst technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of poor conductivity, low specific surface area, and small porosity of Fe-N-C catalysts, and achieve high conductivity and specific surface area Large, the effect of improving the catalytic performance of oxygen reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The method for preparing the Fe-N-CNFs (Carbon nanofibers) catalyst based on Fe-MIL comprises the following steps:
[0064] (1) 1.084g of ferric chloride hexahydrate and 0.664g of terephthalic acid were dissolved in 197ml of N,N-dimethylformamide, and ultrasonically dispersed for 5min to obtain a mixed solution;
[0065] (2) The mixed solution was reacted at a constant temperature of 150° C. for 12 hours. After the reaction was completed, it was centrifuged, washed, and dried at 80° C. for 6 hours to obtain a brown Fe-MIL precursor.
[0066] (3) 0.5g of precursor Fe-MIL was first added to 5ml of N,N-dimethylformamide, ultrasonicated for 30min, then 0.5g of polyacrylonitrile (PAN) was added and stirred at 20°C for 24h to obtain a mixed solution;
[0067] (4) Electrospinning the mixed solution for 5 hours at a voltage of 25kV and a flow rate of 0.5ml / h to obtain nanofibers;
[0068] (5) Pre-fire the nanofibers in an air atmosphere at a heating rate of 5 °C / min to 250 °C ...
Embodiment 2
[0074] The method for preparing the Fe-N-CNFs (Carbon nanofibers) catalyst based on Fe-MIL in this example refers to Example 1, the difference is that the Fe-MIL precursor added in step (3) is 0.75g, and the Fe-MIL precursor The mass ratio to polyacrylonitrile (PAN) is 1.5:1.
[0075] Figure 5 It can be seen that the catalyst contains Fe and Fe 3 C crystals, Fe and Fe coated with nitrogen-doped carbon nanofibers 3 C particles can serve as the active sites of the catalyst.
[0076] Image 6 It can be seen that the degree of graphitization of the catalyst is relatively high, I G / I D =1.06, a high degree of graphitization can improve the electron-conducting ability of the catalyst, thereby improving the catalytic activity of the catalyst.
[0077] Figure 7 It can be seen that the active components of the catalyst, graphitic nitrogen and pyridinic nitrogen, account for a total of 48.7%, graphitic nitrogen can increase the limiting current density, and pyridinic nitrogen ...
Embodiment 3
[0086]The method for preparing the Fe-N-CNFs (Carbon nanofibers) catalyst based on Fe-MIL in this embodiment refers to Example 1, the difference is that the Fe-MIL precursor added in step (3) is 1.0 g, and the Fe-MIL precursor The mass ratio to polyacrylonitrile (PAN) is 2:1.
[0087] Figure 16 It can be seen that the catalyst contains Fe and Fe 3 C crystals, Fe and Fe coated with nitrogen-doped carbon nanofibers 3 The C particle can be used as the active site of the catalyst, which is the same as in Example 2.
[0088] Figure 17 It can be seen that the degree of graphitization of the catalyst is relatively high, I G / I D =0.98, a high degree of graphitization can improve the electron-conducting ability of the catalyst, and then improve the catalytic activity of the catalyst, which is slightly higher than that of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com