Synthesis of tulathromycin
A technology of telamycin and its compound, which is applied in the field of synthesis of telamycin, achieves good industrialization prospects and shortens the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
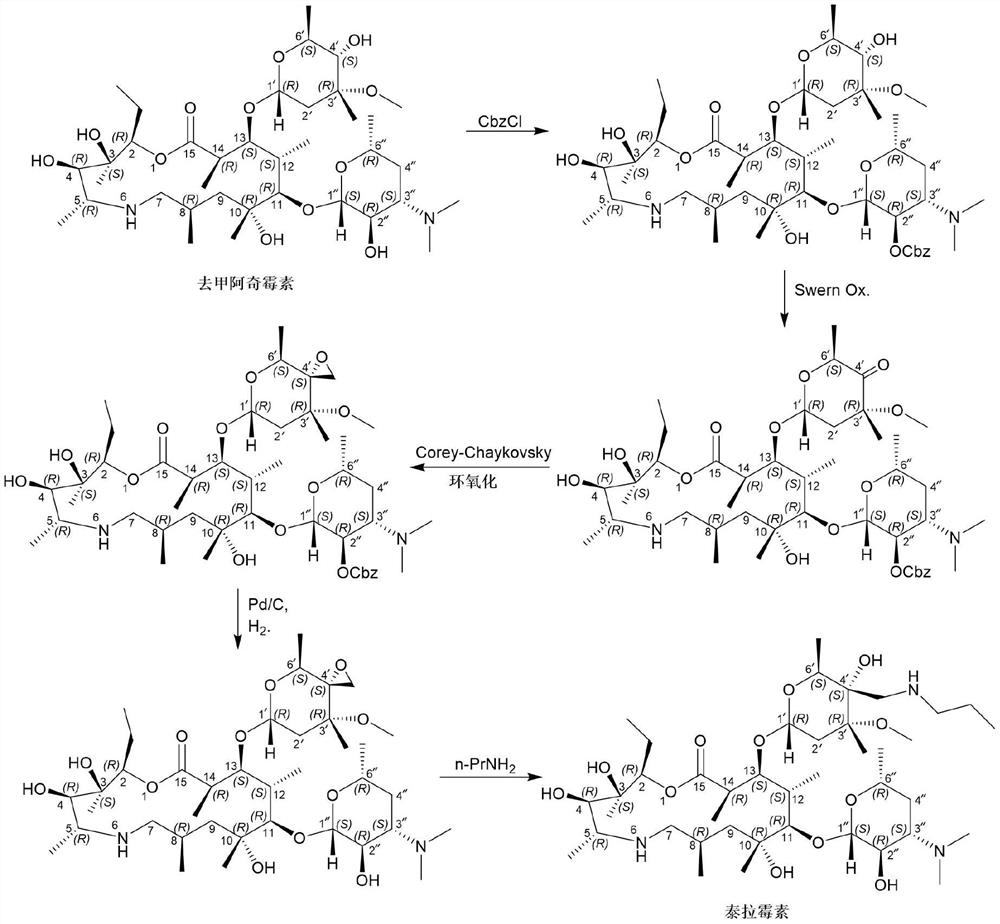
[0021] Embodiment one: the preparation of telamycin
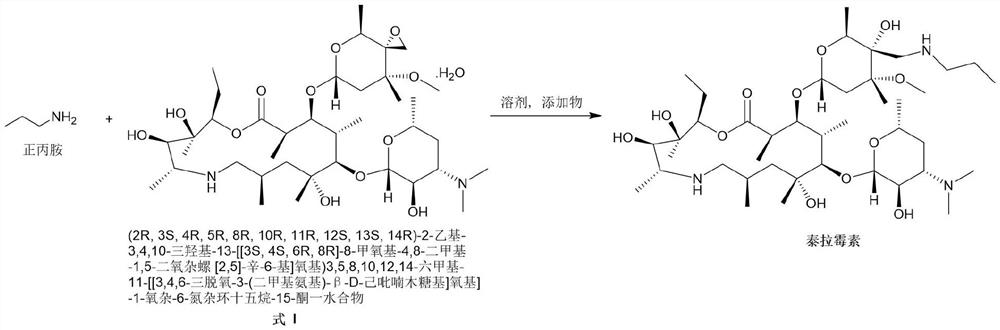
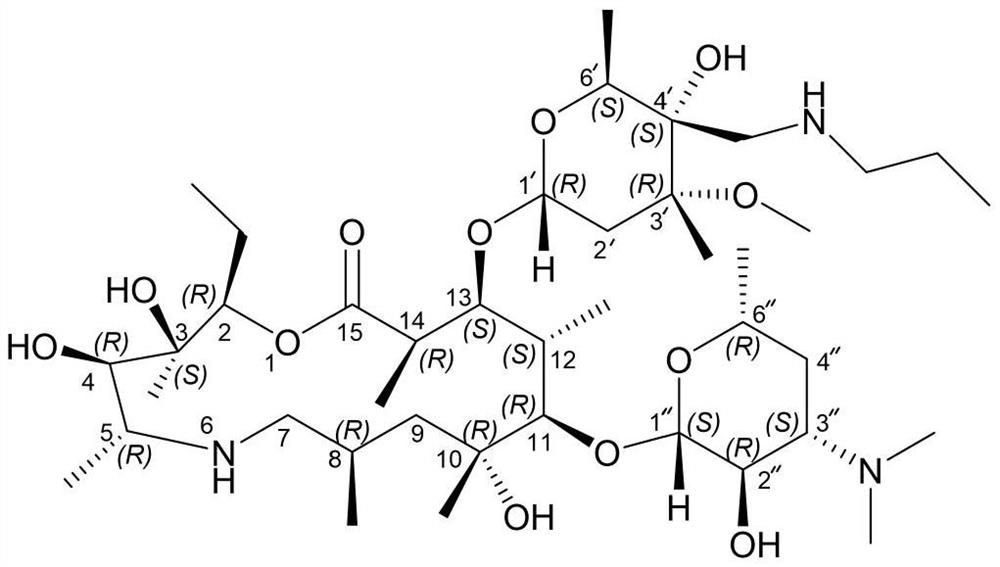
[0022] Add (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-2-ethyl-3,4,10-trihydroxyl-13-[[3S,4S, 6R,8R]-8-methoxy-4,8-dimethyl-1,5-dioxaspiro[2,5]-oct-6-yl]oxy)3,5,8,10, 12,14-Hexamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-hexylopyranosyl]oxy]-1-oxa- 6-Azacyclopentadecane-15-one monohydrate (compound formula I) (110g, 143.8mmol) and isopropanol (500mL) were stirred and dissolved at room temperature. Then n-propylamine (12.75g, 215.7mmol, 1.5eq) and ytterbium trifluoromethanesulfonate (8.9g, 14.4mmol, 0.1eq) were sequentially added to the reaction system. After the addition was complete, the system was heated to 50° C. and stirred for 3 hours. After the reaction is complete, the system is concentrated under high vacuum and reduced pressure to remove the organic solvent, add DMF (450mL) to the residue, heat to 80°C to dissolve, filter while hot, stir the filtrate slowly to about 5°C, stir and crystallize for 2 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com