In-vitro culture method and identification method of micropterus japonicus skeletal muscle satellite cells
A technology of satellite cells and in vitro culture, applied in cell dissociation methods, bone/connective tissue cells, animal cells, etc., can solve problems such as research gaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] In the present embodiment, the specific steps of culturing skeletal muscle satellite cells of perch are as follows:
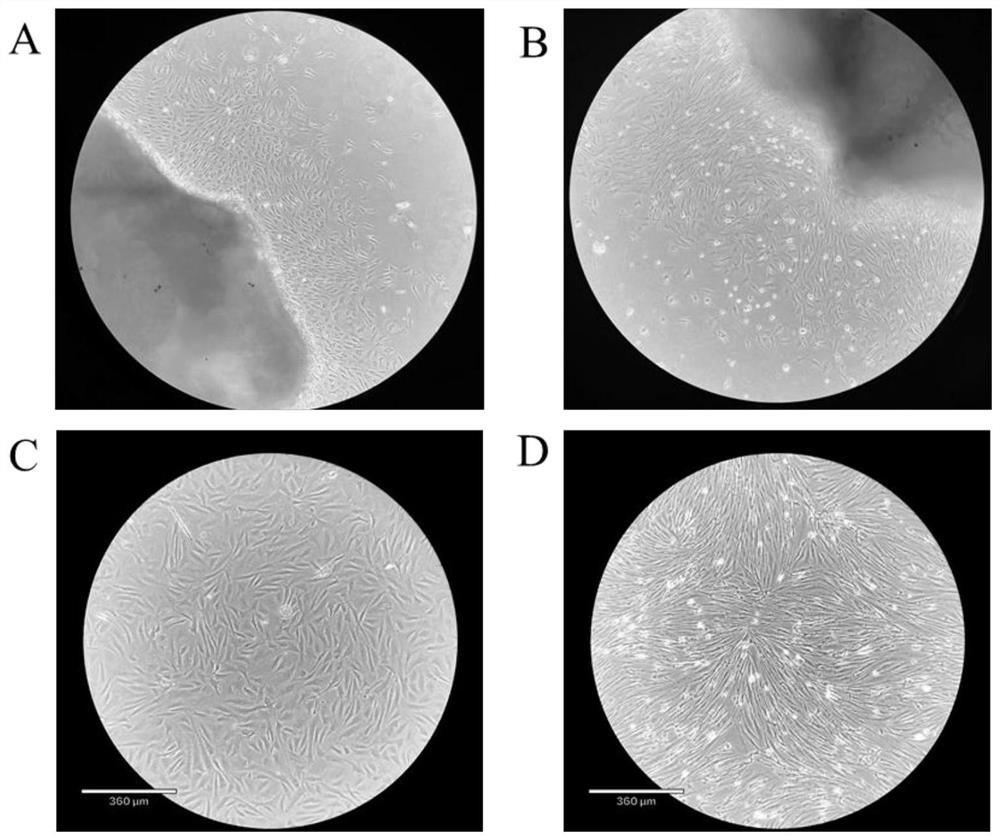
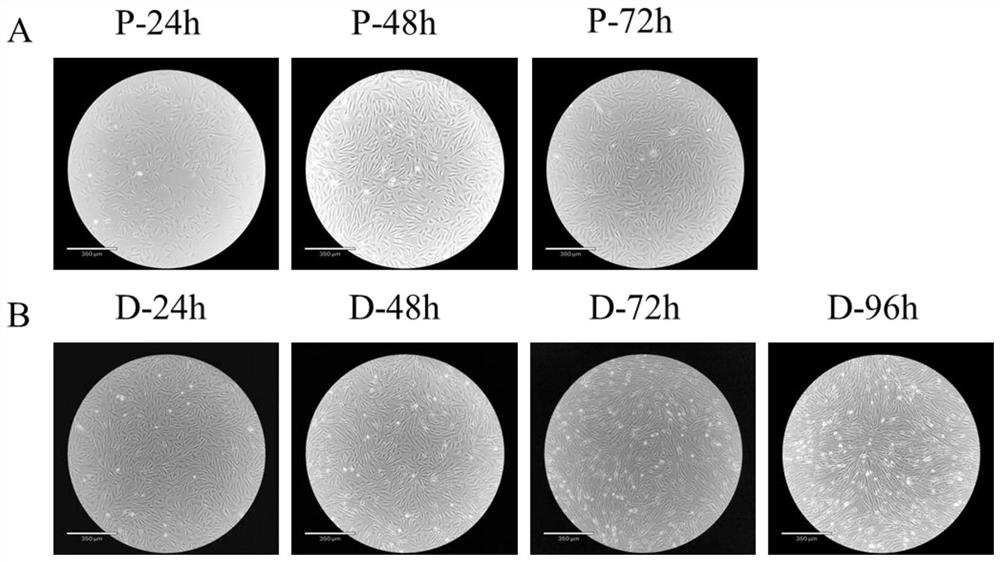
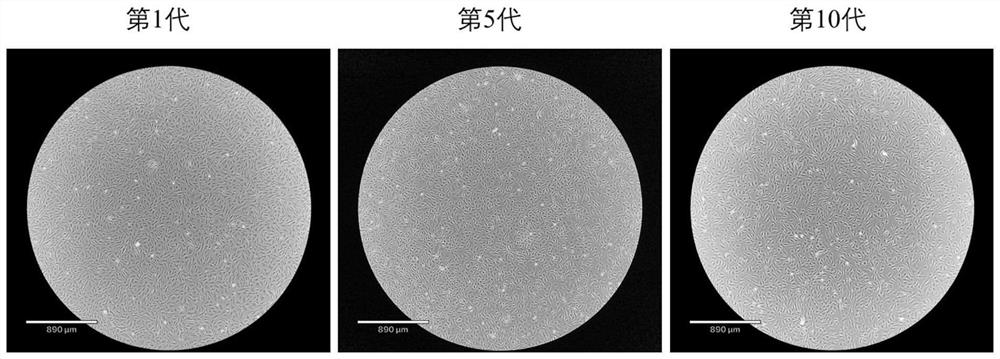
[0057] 1) Primary culture of cells: ① Immerse the individual perch used for sampling in 75% ethanol for 30s, then take a small piece of muscle above the lateral line of the perch and below the dorsal fin, and transfer it into a medium containing 4% third antibody (BI, no. PB180424) in a 50ml sterile centrifuge tube of PBS (Biosharp, BL601A). Transfer the tissue pieces to a sterile 50ml centrifuge tube containing 75% ethanol and soak for 30s, then pass through 50ml centrifuge tubes containing 4%, 3%, 2%, and 1% third-antibody PBS in turn, soak for 5min each time, and shake the centrifuge tube during , so that the tissue block and PBS are fully contacted and sterilized; ② Move the tissue block to a container containing 1% third-antibody PBS; cut the tissue block to about 1mm 3 ; ③ Leave the tissue block solution after step ② to be shredded, discard the up...
Embodiment 2
[0063] Example 2 Identification method of sea bass skeletal muscle satellite cell types and stages
[0064] The method for identification of the cell types cultured in Example 1. In this example, muscle satellite cells before induction of differentiation and cells fused into myotubes after induction of differentiation were collected. Trizol method (Vazyme, R401-01) was used to extract the total number of cells in two stages. RNA, through the reverse transcription kit (Vazyme, P611-01), 1ug cellular RNA was reverse-transcribed into cDNA, and the marker gene pax7 of skeletal muscle satellite cells, the marker genes myod1 and myod2 of the proliferation stage and the marker genes of the differentiation stage were amplified. The marker gene myogenin is used to determine the cell type and stage. The PCR system is 5uL Mix (Vazyme, P213-01), pax7a, pax7b, myod1, myod2, myogenin upstream and downstream primers 0.4uL each, 1uL cDNA, add water to make up to 10uL, and then perform PCR A...
Embodiment 3
[0068] Example 3 Identification method for the differentiation ability of sea bass skeletal muscle satellite cells
[0069] The method for identifying the differentiation ability of passaged cells obtained: After the cells induced to differentiate for 72 hours were washed 1-2 times with PBS, fixed with 4% paraformaldehyde for 30 minutes, washed with PBS for 5 minutes × 3 times, washed with 0.25% Triton X-100 ( Solarbio, T8200) was permeabilized with PBS for 20 min, washed with PBS for 5 min x 3 times, added 0.5 mg / ml DAPI (Solarbio, C0065) for staining for 2 min, and observed under a fluorescent inverted microscope. It can be seen that multinucleated muscle cells appear, indicating that the cultured muscle satellite cells have good ability to induce differentiation in vitro ( Figure 5 , wherein, A is the light microscope image of the perch muscle satellite cells induced and differentiated for 72 hours, and B is the DAPI staining image of the perch muscle satellite cells induc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap