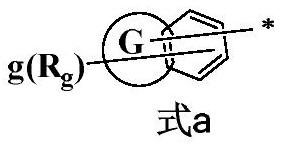
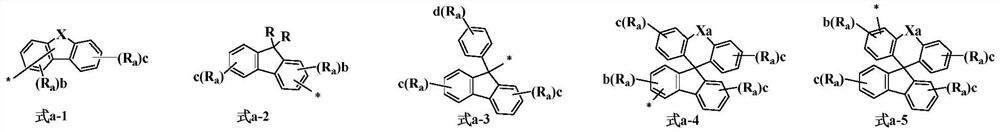
Triarylamine compound, preparation method thereof and organic light-emitting device
A compound, triarylamine technology, applied in the field of organic optoelectronic materials, can solve the problems of low luminous efficiency and service life of organic light-emitting devices, low evaporation temperature, etc., and achieve the effect of improving light extraction efficiency, high purity, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159] The present invention also provides a kind of preparation method of triarylamine compound, described comprises the following steps:
[0160] S1. Under a nitrogen atmosphere, under the action of a palladium catalyst and a base, the primary amine compound a and the intermediate M are subjected to a coupling reaction to obtain the crude intermediate A;
[0161] S2. The crude intermediate A is recrystallized to obtain a purified intermediate A;
[0162] S3. Under nitrogen atmosphere, under the action of palladium catalyst and alkali, the purified intermediate A and the halogen compound c undergo a coupling reaction to obtain the crude compound of formula I, with an HPLC purity of 85% to 95%;
[0163] S4. The crude compound of formula I is subjected to column chromatography and recrystallization to obtain a semi-finished triarylamine compound, and the HPLC purity is greater than or equal to 99%;
[0164] S5. The semi-finished triarylamine compound is sublimated to obtain a ...
Embodiment 1
[0306] [Example 1] Synthesis of compound 4
[0307]
[0308] Synthesis of Intermediate A-1
[0309] Under the protection of nitrogen, toluene (600mL), a-1 (8.46g, 50mmol), M-1 (23.87g, 50mmol), palladium acetate (0.15g, 0.60mmol), tert-butanol were added successively to the 1L reaction flask Sodium (7.69 g, 80 mmol) and tri-tert-butylphosphine (6 mL in toluene). And react under the condition of reflux for 2.5 hours. After the reaction was stopped, the mixture was cooled to room temperature, filtered with diatomaceous earth, the filtrate was concentrated, the residue was recrystallized with toluene, filtered with suction and rinsed with toluene to obtain a recrystallized solid to obtain intermediate A-1 (21.78g, yield 80 %), HPLC detection solid purity >= 99.55%.
[0310] Synthesis of compound 4
[0311] Under nitrogen protection, add toluene solvent (500mL), c-1 (9.56g, 35mmol), intermediate A-1 (19.80g, 35mmol), Pd 2 (dba) 3 (0.41g, 0.45mmol), BINAP (0.37g, 0.60mmol)...
Embodiment 2
[0312] [Example 2] Synthesis of compound 17
[0313]
[0314] Using the same method as in Synthesis Example 1, replace a-1 with equimolar a-2, replace M-1 with equimolar M-2, replace c-1 with equimolar c-2, and synthesize compound 17 (20.03g ), HPLC detection solid purity >= 99.97%. Mass Spectrum m / z: 762.4038 (Theoretical: 762.4022). Theoretical element content (%)C 58 h 42 D. 5 N: C, 91.30; H, 6.87; N, 1.84. Measured element content (%): C, 91.34; H, 6.83; N, 1.80.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com