Traditional Chinese medicine compound external preparation for treating chronic wounds as well as preparation process and use method of traditional Chinese medicine compound external preparation
A technology for chronic wounds and external preparations, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc. sufficient and other problems, to achieve good industrialization prospects, uniform and sufficient dosing, and the effect of being conducive to popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
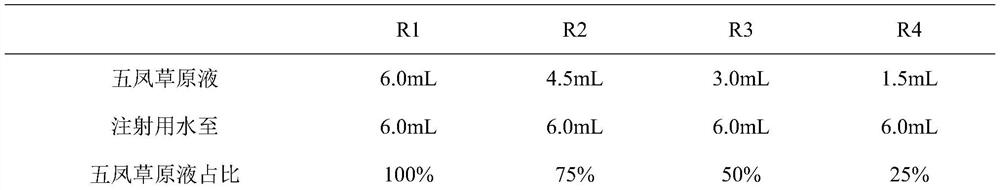
[0027] The viscosity change of the compound Wufeng Grassland Liquid of embodiment 1 different concentrations
[0028] Take the following weight ratio raw materials: Wufengcao 160-240g, Bletilla striata 15-35g, cat's claw grass 20-60g, set aside; soak the above-mentioned raw materials in 8 times the amount of pure water for 1 hour, then add 5 times the amount of water Decoct for 1 hour to obtain the first extract. Then soak the dregs in 5 times the amount of pure water and decoct for 40 minutes to obtain the second extract. Concentrate the first extract and the second extract into one portion, then precipitate for 24 hours, take the supernatant, and continue to heat and concentrate to obtain the compound Wufeng grassland liquid (currently obtained national patent application number: CN201710423274.5 public number CN107184815A) , the crude drug concentration of the prepared compound Wufeng Grassland Liquid is 2g / ml.
[0029] The concentration of Compound Wufeng Grassland Liqui...
Embodiment 2
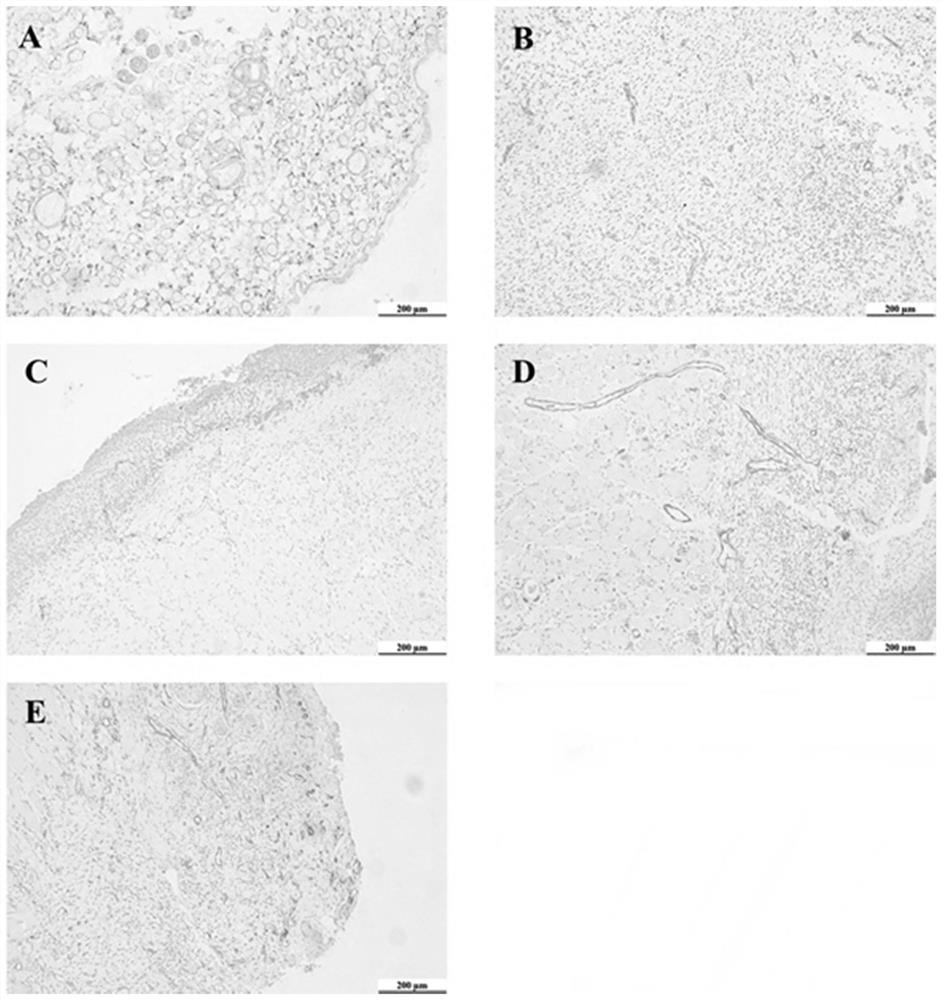
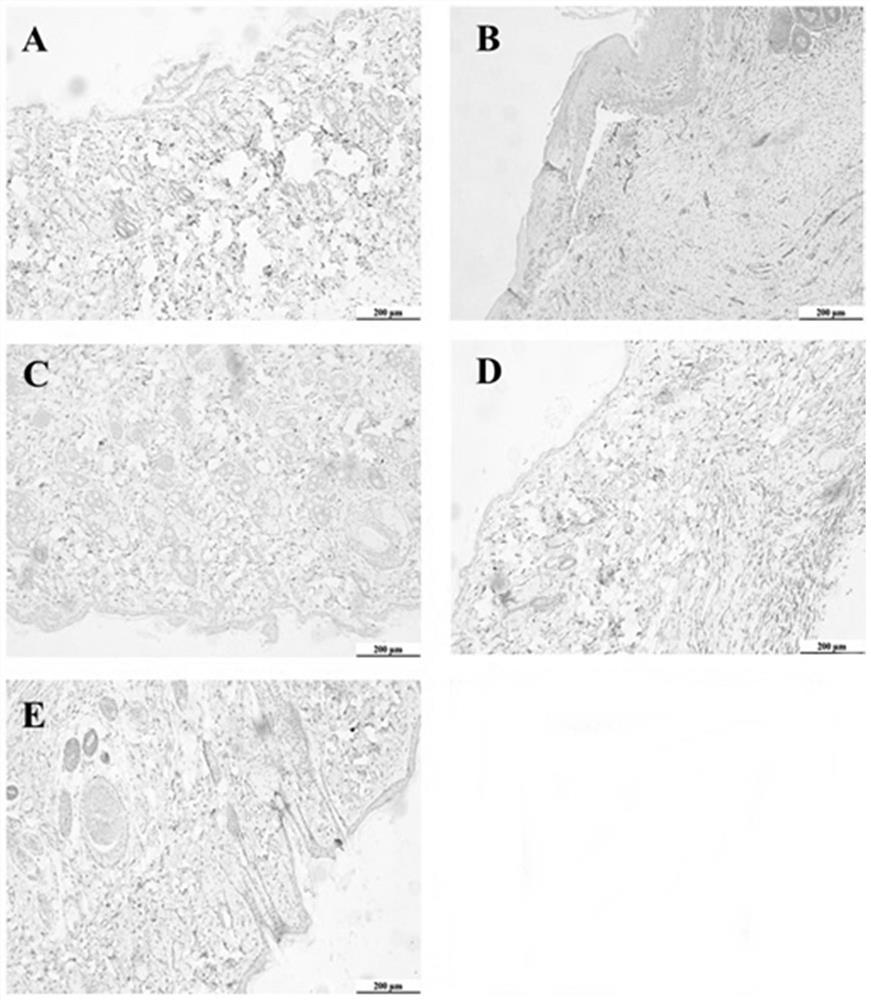
[0034] Example 2 Comparison of Curative Effects of External Application of Compound Wufengcao Liquid in Treating 96 Rats with Venous Ulcer Wounds of Lower Limbs
[0035] (1) Experimental objects and groups:
[0036] 96 SD rats (permit number: SCXK (Zhejiang) 2019-0002) provided by the Qinglongshan Animal Farm in Jiangning District, Nanjing, half male and half female, weighing (250±20) g. After successfully establishing the rat model of venous ulcer of lower extremities, 80 rats (half male and half male) were randomly divided into 6 groups, 16 rats in each group. These groups are model group, control group, Chinese medicine preparation group I (use R1 prescription preparation), Chinese medicine preparation group II (use R2 prescription preparation), Chinese medicine preparation III group (use R3 prescription preparation), and Chinese medicine preparation IV group (use R4 prescription preparation). prescription preparations)
[0037] (2) Local wound drug delivery
[0038] 1) ...
Embodiment 3
[0057] Embodiment 3 Formulation prescription fumbles -- the selection of suspending agent
[0058] According to the research of Example 1 and Example 2, a preliminary prescription of the compound Wufengcao suspension was designed, as shown in Table 3, involving prescriptions R5-R13.
[0059] Table 3 Prescription of compound Wufengcao suspension
[0060]
[0061]
[0062] The appearance and sedimentation volume ratio of the above-mentioned prescriptions after long-term storage at room temperature for 60 days were further investigated, and the specific results are shown in Table 4 and Table 5.
[0063] Table 4 Prescription (R5-R8) 60-day storage inspection results at room temperature
[0064]
[0065] Table 5 Prescriptions (R9-R13) placed at room temperature for 60 days to investigate the results
[0066]
[0067] From the above results, it can be seen that: (1) In the prescriptions R5, R6, and R7 with glycerin as the suspending agent, the proportion of glycerin wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com