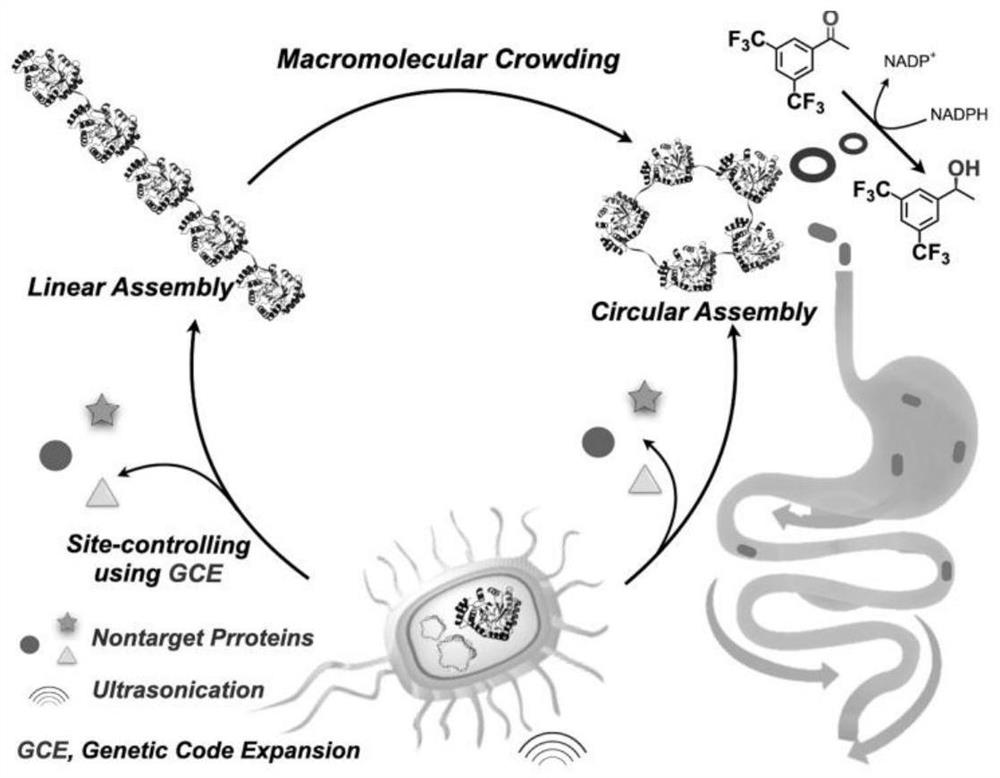
Method for preparing stable zymoprotein ring through precise regulation and control assembly
An enzyme protein and stabilization technology, applied in the direction of enzyme stabilization, oxidoreductase, recombinant DNA technology, etc., can solve the problems of unpredictability and lack of ligand, and achieve the effect of separation, good catalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method of preparing a stable enzyme protein regulation ring assembly precision, includes the following steps:
[0037] (1) E. coli (MG1655) becomes host gene expression induced keto reductase, and the cells were harvested by centrifugation to obtain the precipitate is centrifuged 8000 rpm for the number of revolutions, time of 5min, and washed with PBS buffer (0.02mol·L -1 , PH 7.0) was washed precipitate; pellet was resuspended in PBS and lysed by sonication cell, wherein an amount of PBS was added 1 / 5 volume of the original cell suspension, ultrasonication using an ice bath, 400W power, crushing time 10min, wherein each of 10s set stop 7S broken 3s; soluble and insoluble fraction after cell disruption by centrifugation, also, the number of revolutions of 10000 rpm for centrifugal, time 15min, such isolated cell homogenate supernatant;
[0038] (2) the crosslinking agent bis alkyne (5,6,11,12-tetrahydro-dibenzo [a, e] cyclooctene was dissolved in isopropanol, at a concen...
Embodiment 2
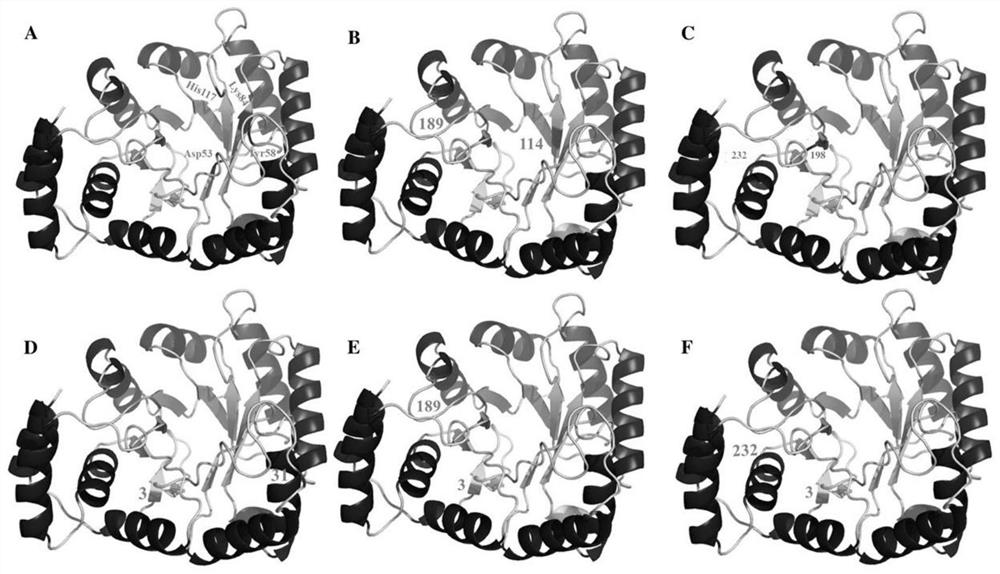
[0044] Two mutant AKR incorporated unnatural amino acid sites of regulation of the morphology of enzyme protein assembly
[0045] The three-dimensional view AKR gene, the active site of the gene is Asp53, Tyr58, Lys84 and His117, select the appropriate mutation sites need to avoid the active site and the active site of NADP +. The present invention is selected six sites away from the active site and the active site, and then obtained 2.5 AKR different spatial distance mutants, a three-dimensional map of the mutation site mutants such two AKR figure 2 Distance figure 2 A is the active site of the gene is Asp53, Tyr58, Lys84 and His117, figure 2 B-F, respectively, represent the distance between the AKR-114Y-189Q, AKR-198Y-232W, AKR-3Y-31Y, AKR-3Y-189Q, three-dimensional representation gene AKR-3Y-232W, and the two mutation sites. AKR points based on different mutants, under microwave radiation, to help with the crosslinking agent diacetylene AKR two mutants, crosslinked morphology o...
Embodiment 3
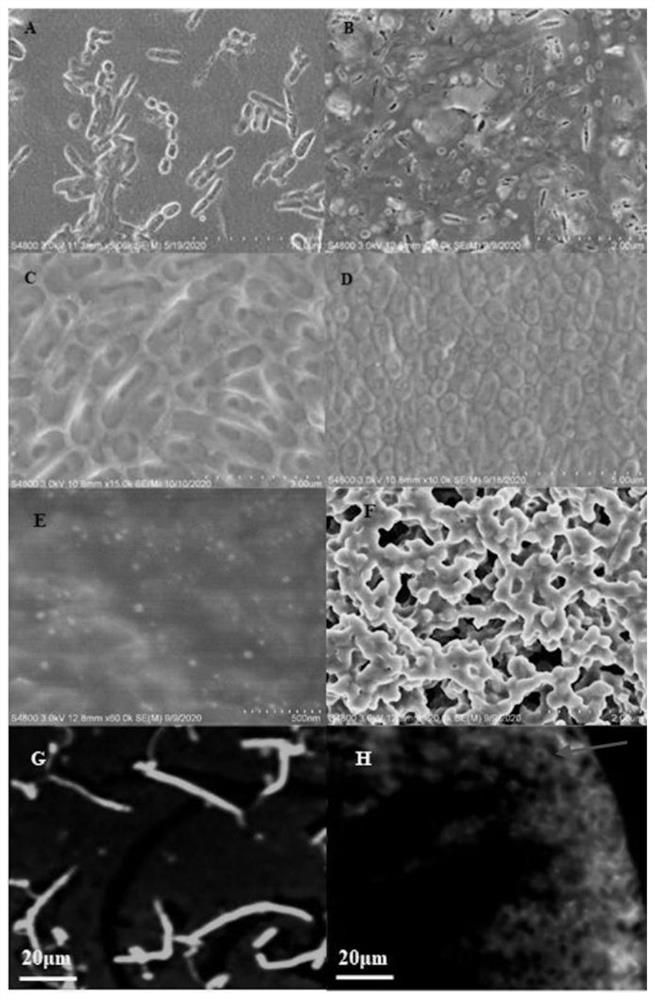
[0050] Obtained by different methods annular assembly
[0051] Concentration on the introduction of functions and mechanisms of protein covalently assembled in AKR-114-189, can be easily assembled into a rod, and a linear strip. Using different concentrations of enzyme formed SEM AKR-114-189 assembly, TEM and CLSM as FIG. Figure 4 Shown, respectively, when using six different concentrations (5.02mg · mL -1 , 16.04mg · mL -1 , 32.16mg · mL -1 , 40.20mg · mL -1 , 60.13mg · mL -1 ) Of the enzyme protein AKR-114-189 covalent crosslinking assembly, with increasing protein concentration, are also more likely mutant AKR form a ring ( Figure 4 A, 4B, 4C, 4D). When considering the increased protein concentration, increased chance of collision between molecules, a greater chance of cyclization, resulting in formation of a protein loop. Thus, depending on the enzyme protein was added to an unnatural amino acid azide specific covalent binding can regulate and control the behavior of the prote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com