Application of small-molecule inhibitor in prevention and treatment of respiratory viral pneumonia
A viral pneumonia and inhibitor technology, applied in clinical, biological, and medical fields, can solve problems such as uncontrollable progress and patient death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. Kyn significantly induces ACE2 expression through the AHR pathway
[0055] 1. Experimental steps:
[0056] 1. Prepare reagents: Ca-free 2+ / Mg 2+ HBSS+5%FBS+2mM EDTA+collagenase+hyaluronidase, preheated to 37°C; sacrifice the mice, cut the lungs; remove the bronchi and lymph nodes; use Ca-free 2+ / Mg 2+ Wash the contents of the lungs with PBS; cut the lungs into as small pieces as possible, put them into a 50mL centrifuge tube, add 20mL of the reagent prepared in the first step; shake on a horizontal shaker at 37°C and 250rpm for 20min; filter on a 200-mesh filter Transfer the supernatant to a new 50mL centrifuge tube, and add 20mL of the reagent prepared in the first step to the remaining intestinal tissue; shake on a horizontal shaker at 37°C and 250rpm for 20min; filter the supernatant through a 200-mesh filter and transfer it to the previous 50mL centrifuge tube; filtrate After centrifugation at 400 g for 5 min, the obtained cell pellet was lung epith...
Embodiment 2
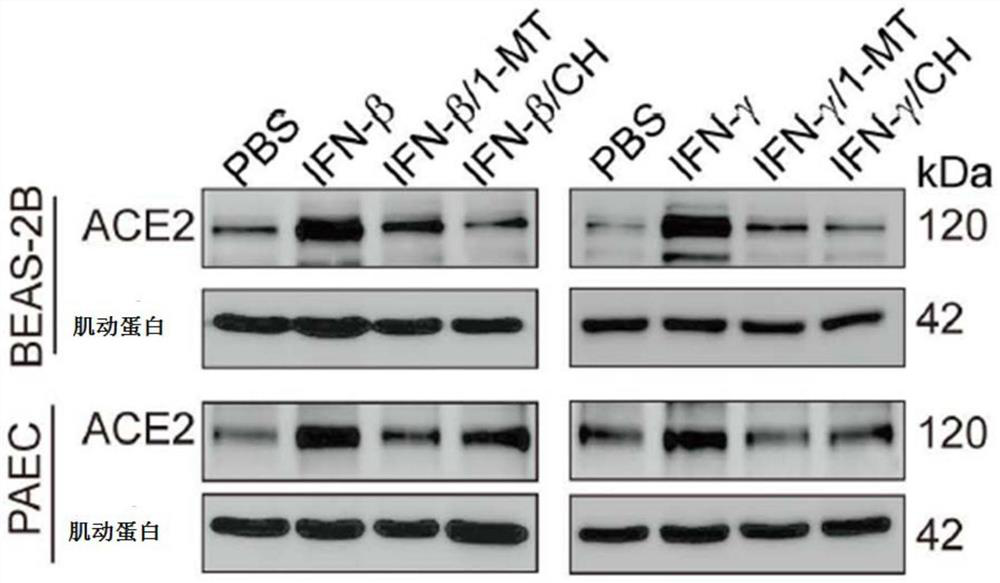
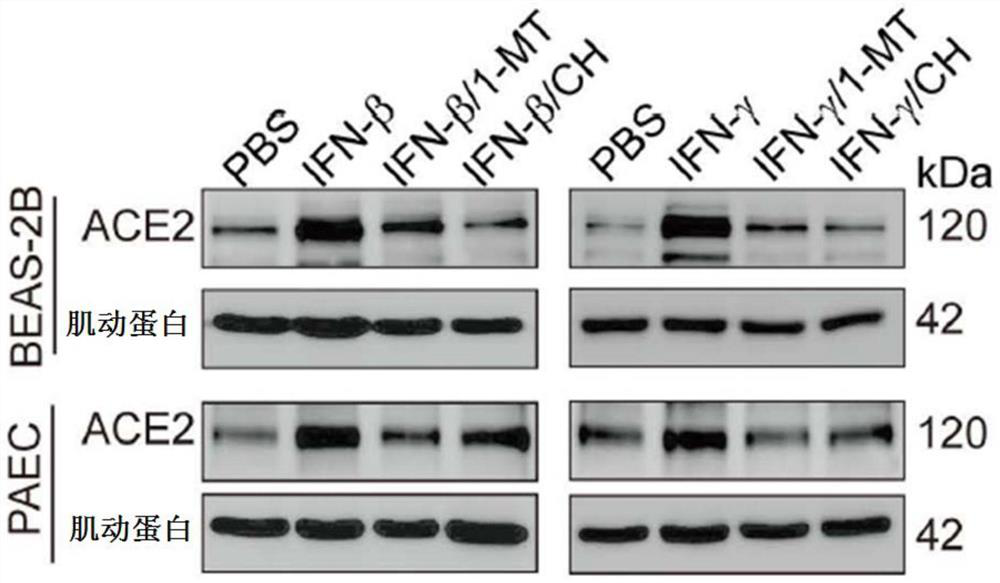
[0067] Example 2. Interferon β induces ACE2 expression through the IDO-AHR pathway
[0068] 1. Experimental steps
[0069] 1. Prepare reagents: Ca 2+ / Mg 2+ HBSS+5%FBS+2mM EDTA+collagenase+hyaluronidase, preheated to 37°C; sacrifice the mice, cut the lungs; remove the bronchi and lymph nodes; use Ca-free 2+ / Mg 2+ Wash the contents of the lungs with PBS; cut the lungs into as small pieces as possible, put them into a 50mL centrifuge tube, add 20mL of the reagent prepared in the first step; shake on a horizontal shaker at 37°C and 250rpm for 20min; filter on a 200-mesh filter Transfer the supernatant to a new 50mL centrifuge tube, and add 20mL of the reagent prepared in the first step to the remaining intestinal tissue; shake on a horizontal shaker at 37°C and 250rpm for 20min; filter the supernatant through a 200-mesh filter and transfer it to the previous 50mL centrifuge tube; filtrate After centrifugation at 400 g for 5 min, the obtained cell pellet was lung epithelial c...
Embodiment 3
[0074] Example 3. Interferon gamma induces ACE2 expression through the IDO-AHR pathway
[0075] 1. Experimental steps
[0076] 1. The operation of mouse alveolar cell isolation is the same as that described in Example 1 above.
[0077] 2. Divided into the following groups: control group (PBS treatment), interferon gamma group (10ng / ml INF gamma treatment for 48 hours), interferon gamma group plus IDO inhibitor (10ng / ml INF gamma and 0.2mM 1-MT treatment for 48 hours ) and interferon γ group plus AHR inhibitor (10ng / ml INFγ and 4μM CH223191 treated for 48 hours).
[0078] 3. The operations of Western blot detection, BCA protein quantification, gel plate preparation, SDS-PAGE electrophoresis, membrane transfer, antibody incubation, and chemiluminescence imaging were the same as those described in Example 1 above.
[0079] 2. Experimental results
[0080] Western blotting showed that when BAES-2B and mouse type II alveolar epithelial cells were treated with interferon gamma, i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com