Tropane alkaloid (TA) producing non-plant host cells, and methods of making and using the same
A technology for alkaloids and tropane, which is applied to non-plant host cells producing tropane alkaloids (TA) and the fields of their preparation and use, can solve the problems of long generation time, poor economies of scale, unproven and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0269] For high-purity, small-scale preparations, TA precursors or TA can be purified in a single step by liquid chromatography.
[0270] Yeast-derived alkaloid APIs versus plant-derived APIs
[0271] Clarified yeast medium (CYCM) may contain various impurities. The clarified yeast medium can be dehydrated by vacuum and / or heat to produce an alkaloid-rich powder. This product is similar to a concentrate of nightshade leaves (CNL), which are used by active pharmaceutical ingredient (API) manufacturers to extract tropane alkaloids subject to further chemical treatment and purification. For purposes of the present invention, CNL is a representative example of any type of purified plant extract from which one or more desired alkaloid products may ultimately be further purified. Table 5 highlights impurities in these two products that may be specific to CYCM or CNL, or may be present in both. By analyzing products of unknown origin for a subset of these impurities, one skilled i...
Embodiment 1
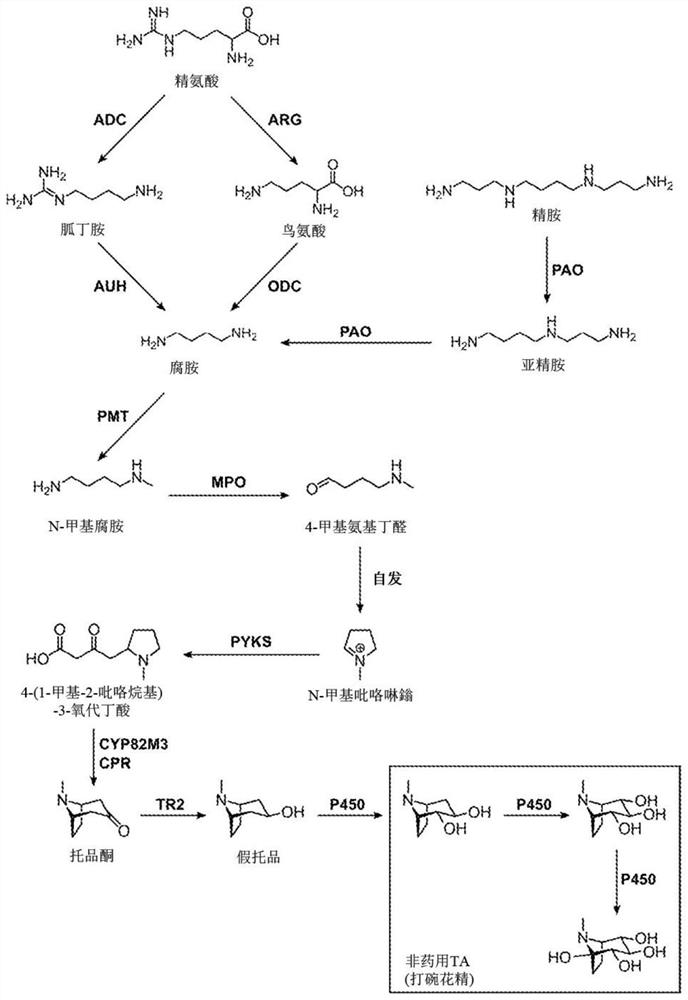
[0311] Example 1. Platform yeast strains engineered for high-level putrescine production
[0312] The tropin portion of TA is derived from the amino acid arginine via the polyamine molecule putrescine. With the aim of increasing intracellular concentrations of TA precursor molecules including putrescine, NMP, 4MAB and NMPy, S. cerevisiae strains with increased flux through the arginine and polyamine biosynthetic pathways were developed. These strains combined genetic modifications for increasing carbon and nitrogen flux from central metabolism to arginine and polyamine biosynthesis in general, and included the introduction of key heterologous enzymes to additionally produce the TA precursor putrescine. Employing genetic modifications, including feedback inhibition of genes encoding native biosynthetic enzymes and regulatory proteins, introduction of mitigation mutations, fine-tuning of transcriptional regulation of native biosynthetic enzymes, deletion or disruption of genes e...
Embodiment 2
[0326] Example 2. Engineering Yeast Strains for the Production of NMPy
[0327] A strain of S. cerevisiae was developed by modifying the putrescine-overproducing strain developed in Example 1 for the production of the TA precursor NMPy. These strains combined genetic modifications aimed at increasing carbon and nitrogen flux from putrescine to NMPy biosynthesis and included the introduction of key heterologous enzymes to produce the TA precursors NMP, 4MAB, and NMPy. Genetic modifications were used, including modification of the N- and / or C-terminal domains of the enzyme of interest to improve activity in a heterologous host, and deletion or disruption of the gene encoding the enzyme that drives the precursor molecule away from the intended pathway .
[0328] 2.1) The biosynthetic pathway in the engineered strain is capable of producing NMPy from endogenous putrescine. Putrescine is first converted to N-methylputrescine (NMP) by SAM-dependent N-methyltransferase (PMT), which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com