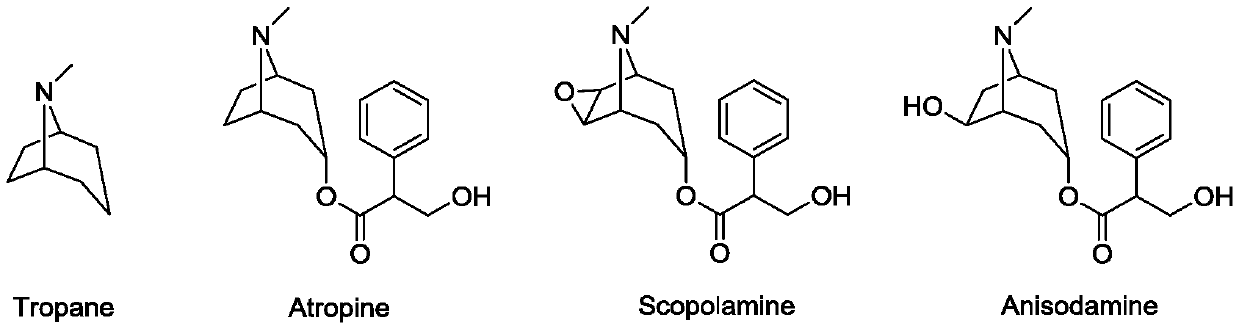
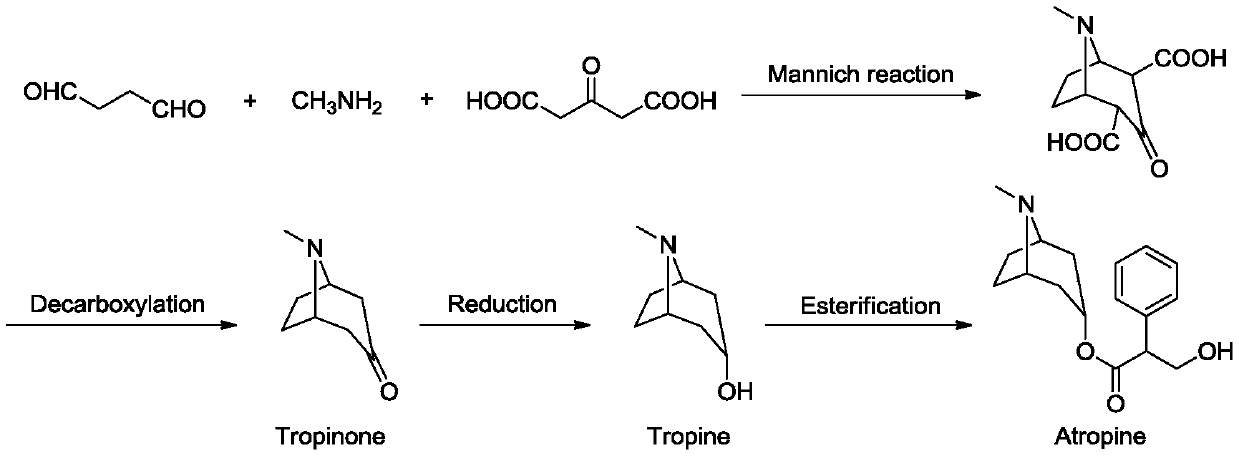
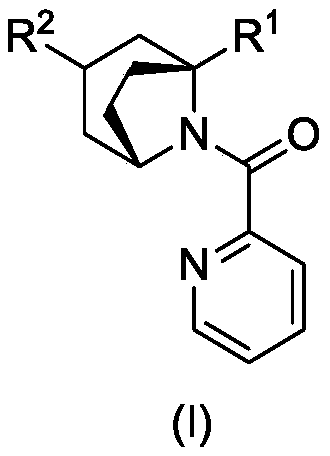
A kind of tropane type alkaloid and its synthetic method
A synthesis method and compound technology, applied in the fields of drug combination, organic chemistry, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] (1) Preparation of compound 3:
[0024]
[0025] Operational references: Gong Chen; Gang He. Angew. Chem. Int. Ed., 2011, 50, 5192–5196.
[0026] (2) Preparation of compound 5:
[0027]
[0028] The procedure was as follows: 1-Aminocycloheptanoic acid (560 mg, 3.56 mmol) and 4 mL of methanol were added to a 15 mL round bottom flask and stirred in an ice bath. Thionyl chloride (0.8 mL, 11 mmol) was added dropwise thereto, gradually returned to room temperature, and reacted overnight. After the reaction, evaporate to dryness under reduced pressure, add a small amount of petroleum ether and filter, and wash the solid several times with a small amount of petroleum ether to obtain a white solid, namely 1-aminocycloheptyl ester hydrochloride. The resulting 1-aminocycloheptyl ester hydrochloride, picolinic acid (529 mg, 4.3 mmol), EDCI (1.04 mg, 5.4 mmol), DMAP (50 mg, 0.4 mmol) and dichloromethane (5 mL) were added to a 10 mL round bottom flask , the reaction was sti...
Embodiment 1
[0032] The preparation of embodiment 1 compound 4:
[0033]
[0034] The operation is as follows: at room temperature, 2-pyridinecarboxylic acid protected cycloheptylamine 3 (ie compound 3) (21.8mg, 0.1mmol), PdCl 2 (1.8mg, 0.01mmol), Ag 2 CO 3 (55.2mg, 0.2mmol), 4-Iodonitrobenzene (49.8mg, 0.2mmol), 2,6-Dimethoxybenzoic acid (36.4mg, 0.2mmol), Na 3 PO 4 (41.0mg, 0.25mmol) and TCE (1mL) were added into a 10mL microwave reaction tube with a power of 20W and reacted at 140°C for 2 hours. After the reaction was completed, it was naturally cooled to room temperature, filtered through celite, and spin-dried. Using petroleum ether: ethyl acetate = 2:1 as a developing solvent, 19.1 mg of the target compound 4 was obtained through preparative plate separation, with a yield of 88%. 1 H NMR (400MHz, CDCl 3 )δ8.57(d,J=4.6Hz,1H),7.85–7.66(m,2H),7.37–7.27(m,1H),4.84(s,1H),4.57(s,1H),2.06–1.72 (m,7H), 1.66–1.52(m,2H),1.51–1.41(m,1H); 13 C NMR (100MHz, CDCl 3 )δ164.1, 154.6, 148....
Embodiment 2
[0035] The preparation of embodiment 2 compound 6:
[0036]
[0037] The operation is as follows: at room temperature, the 2-pyridinecarboxylic acid protected cycloheptylamine derivative 5 (ie compound 5) (27.6mg, 0.1mmol), PdCl 2 (1.8mg, 0.01mmol), Ag 2 CO 3 (55.2mg, 0.2mmol), 2-Iodobenzonitrile (45.8mg, 0.2mmol), 2,6-Dimethoxybenzoic acid (36.4mg, 0.2mmol), Na 3 PO 4 (41.0mg, 0.25mmol) and TCE (1mL) were added into a 10mL microwave reaction tube with a power of 20W and reacted at 140°C for 2 hours. After the reaction was completed, it was naturally cooled to room temperature, filtered through celite, and spin-dried. Using petroleum ether: ethyl acetate = 2:1 as the developing solvent, 12.0 mg of the target compound 6 was obtained by separation through a preparative plate, with a yield of 44%. 1 H NMR (400MHz, CDCl 3 )δ8.57(d,J=4.1Hz,1H),7.92–7.68(m,2H),7.35(t,J=5.1Hz,1H),5.20–5.05(m,1H),3.73(s,3H ),2.43–2.22 (m,3H),2.05–1.92(m,2H),1.85–1.68(m,3H),1.53–1.34(m,2H); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com