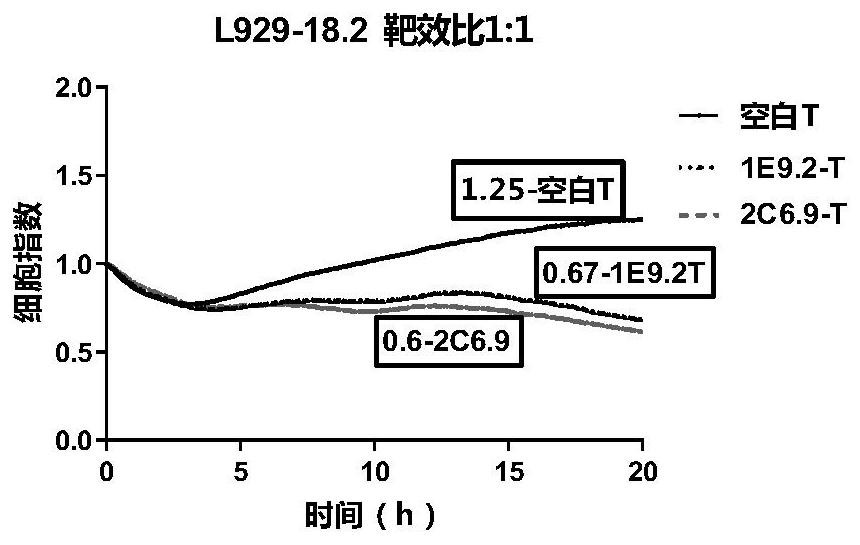
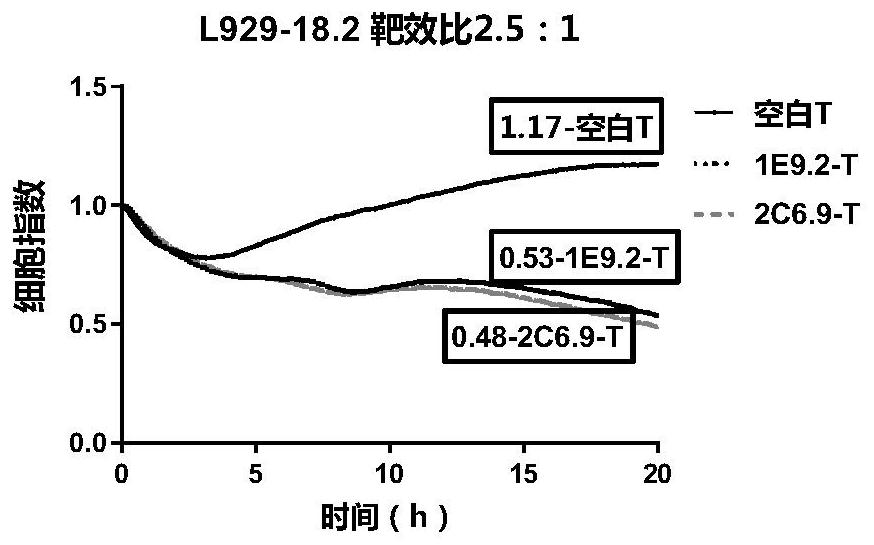
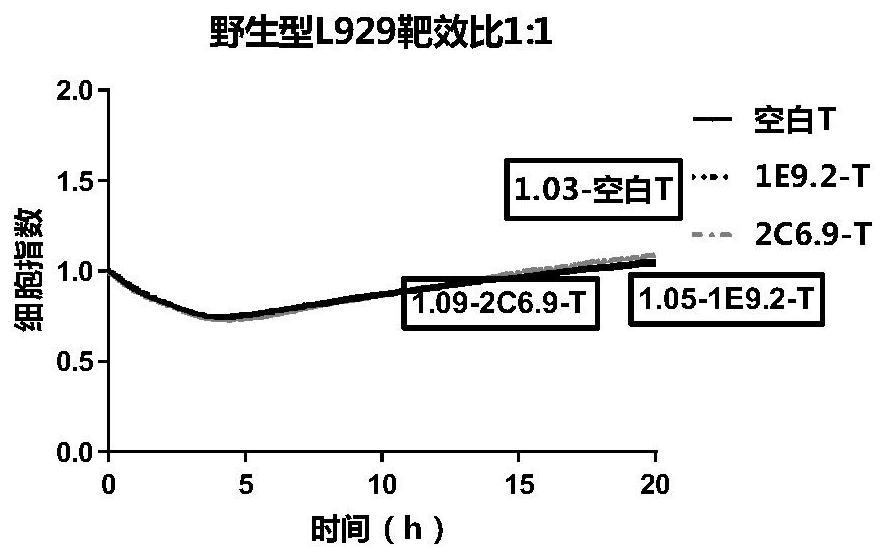
CLDN18.2-targeted chimeric antigen receptor and composition and application of CLDN18.2-targeted chimeric antigen receptor
A chimeric antigen receptor and antigen technology, applied in the field of biomedicine, can solve the problems of high mortality, poor prognosis, and limited treatment options for advanced or recurrent gastric cancer, and achieve good specificity, good animal tolerance, and inhibition The effect of tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0320] Example 1: Preparation of Antibody Targeting Human Claudin 18.2scFv
[0321] Two murine anti-CLDN18.2 monoclonal antibodies 1E9.2 and 2C6.9 were screened by hybridoma technology. The above murine monoclonal antibody was analyzed to obtain its CDR-H1, CDR-H2, CDR-H3, CDR-L1, CDR-L2, CDR-L3, VH, VL sequences (see sequence information table). Then carry out the above method of humanization to finally obtain the humanized antibody 1E9.2-HZ11 (its VH and VL are respectively shown in SEQ ID NO: 13 and 14); 2C6.9-HZ21 (its VH and VL are respectively shown in SEQ ID NO:16 and 17). The sequence of CDR-H2 of 2C6.9-HZ21 was further modified to the sequence shown in SEQ ID NOs:37 or 53.
[0322] The VH and VL of the above humanized antibody were linked by a linker (SEQ ID NO: 44) to obtain scFv, and the sequence information of each scFv is shown in the table below.
[0323] Table 2: Structure of scFv
[0324]
[0325]
Embodiment 2
[0326] Example 2: Construction and preparation of chimeric antigen receptor (CAR) lentiviral expression vector
[0327] 1) Construction of lentiviral plasmid:
[0328] Based on the scFv sequence in the above examples and the reference T scFv sequence (the reference T is derived from the document J NatlCancer Inst. 2019Apr 1; 111(4):409-418, which is hu8E5 in the document), further construct CAR lentiviral expression carrier. Using the intracellular domain of CD137 and the ITAM region of CD3Zeta as the activation signal, fuse with the above scFv, add signal peptide, CD8 hinge region, CD8 transmembrane region, use primer F (SEQ ID NO:54) and primer R (SEQ ID NO: 55), construct a chimeric antigen receptor expression vector, and the structure of the constructed chimeric antigen receptor is shown in Table 3 below.
[0329] Table 3: Structures of Chimeric Antigen Receptors
[0330]
[0331] 2) Virus packaging:
[0332] Add the above-constructed CAR lentiviral plasmid and tr...
Embodiment 3
[0333] Example 3: Construction and preparation of co-expression CAR lentiviral expression vector
[0334]On the basis of the CAR structure in Example 2, other biologically active molecules (such as PD-1 scFv, whose nucleotide sequence is shown in SEQ ID NO: 72, its upstream P2A self-cleavage peptide nucleotide sequence is SEQ ID NO: 69; or mIL-15, its nucleotide sequence is shown in SEQ ID NO: 73, its upstream P2A self-cleavage peptide nucleotide sequence The sequence is the sequence of SEQ ID NO: 70) to obtain co-expressed CAR. The lentiviral expression vector co-expressing CAR was prepared by the same method as in Example 2. When the above-mentioned co-expressed CAR is expressed in cells, it will be cleaved from the PGP in the P2A sequence, so that the linked biologically active molecules are secreted outside the CAR-T cell or chimerically expressed on the CAR-T cell membrane, synergistically exerting anti-tumor effects. effect. For example, since CLDN18.2 CAR-T specifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com