Estrogen-related receptor beta mutant and application thereof
A technology of estrogen and mutants, applied in the fields of genetic engineering and protein engineering, can solve the problems that hinder the research of ERRβ target drugs, and it is difficult to obtain soluble ERRβLBD protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1, bioinformatics analysis to explore and determine mutation sites.
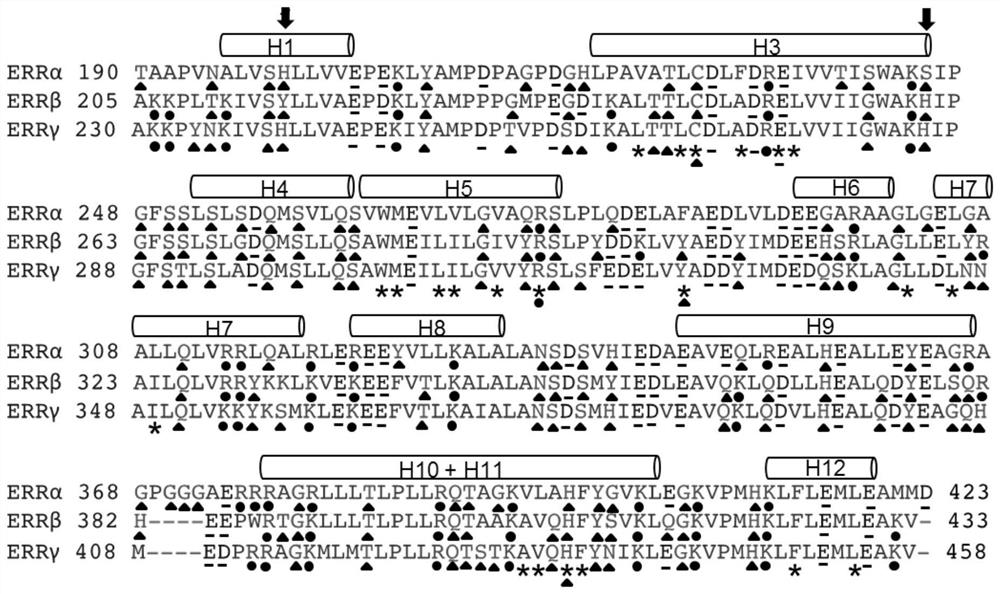
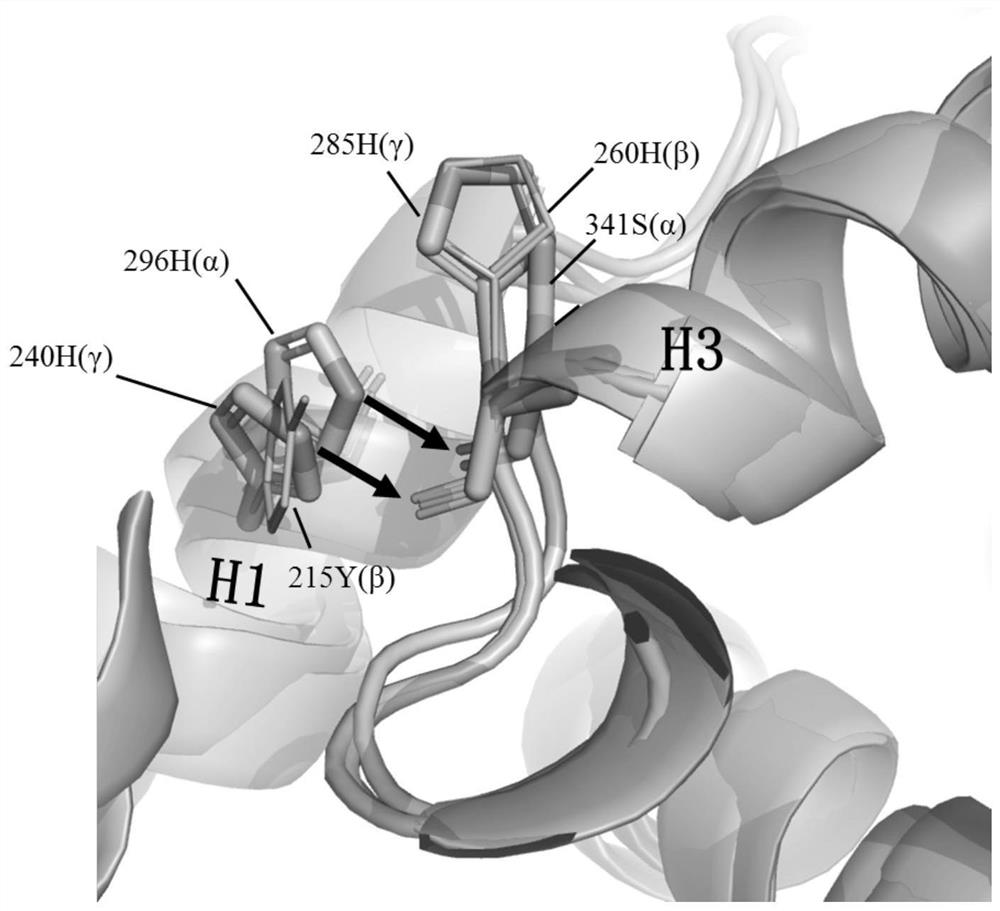
[0041] In order to identify the key residues that may lead to the low solubility and poor stability of ERRβ LBD, bioinformatics analysis was performed on ERRβ and ERRα and ERRγ with high solubility and stability, including primary sequence alignment, 3D structure alignment and mutation model simulation . Such as figure 1 , based on the sequence alignment, it was found that the 215th amino acid of ERRβ LBD helix 1 (H1) is tyrosine (Y215), while the other two members of ERR, ERRα and ERRγ, are both histidine here, and histidine is more than tyrosine Amino acids are more hydrophilic and charged. Such as figure 2 , further analysis of the three-dimensional structure of ERRα (PDB: 2e2r) and ERRγ (PDB: 1kv6) found that the histidine in H1 here plays an important role in maintaining the stability of ERRα and ERRγ. In ERRα and ERRγ, the imidazole side chain of histidine here forms hydrogen bonds ...
Embodiment 2
[0042] Example 2, using point mutation technology to construct a mutant expression plasmid.
[0043] See SEQ ID NO.1 for the amino acid sequence of human ERRβ (UniProtKB identification number: O95718-3). In the present invention, firstly, the prokaryotic expression plasmid vector pET24a (Novagen, USA) is used, and the wild-type ERRβ sequence is used as a template, and the designed primers containing mutations are cloned into the ERRβ gene by overlapping extension PCR. Taking the Y215H mutation as an example, the primers used are:
[0044] F: 5'-cattgaccaagattgtctcacacctactggtggctgagccgga-3'
[0045] R: 5'-tccggctcagccaccagtaggtgtgagacaatcttggtcaatg-3'
[0046] The reaction conditions were pre-denaturation at 95°C for 5 min, followed by 26 cycles of the following program: denaturation at 95°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 6 min. The DNA after the PCR reaction was digested with ThermoFastDigest restriction endonuclease DPN1 to destroy the w...
Embodiment 3
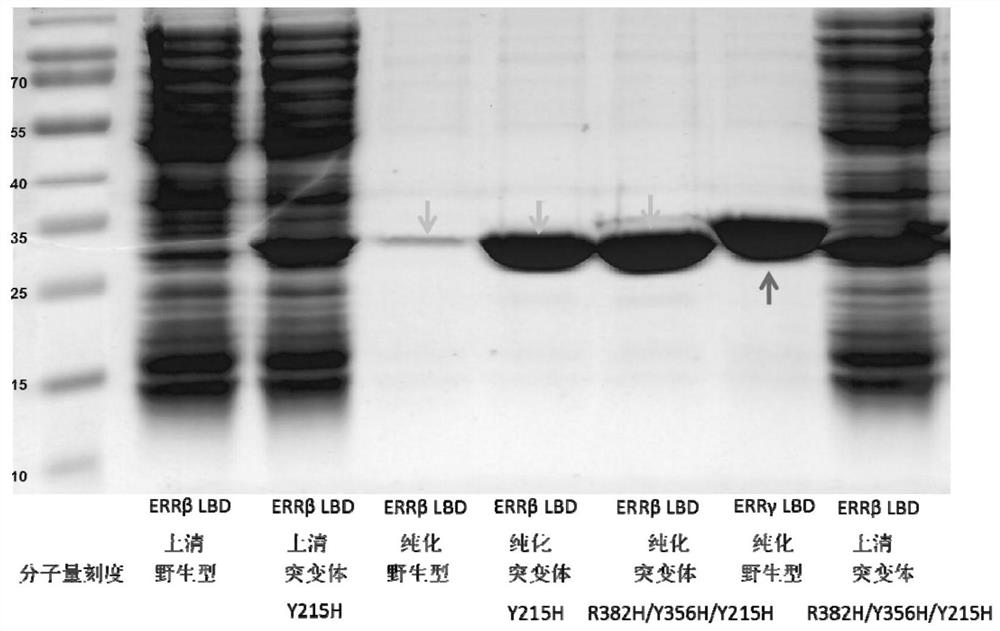
[0059] Example 3, Determination of solubility and expression of ERRβ protein containing Y215H mutation.
[0060] The constructed mutant protein and wild-type protein were expressed and tested in vitro on a small scale. The constructed human pET24a ERRβLBD gene contains a 6 polyhistidine fusion tag. Transform the constructed plasmid into Escherichia coli BL21(DE3), culture and amplify at 30°C, cool down to 18°C when the OD600 is about 0.8, and add a final concentration of 0.1mM isopropyl 1-thio-β -D-galactoside (IPTG) induced target protein expression overnight. The expressed E. coli cells were collected after low-speed centrifugation (4200 r.p.m.) at 4°C for 30 min, and resuspended in buffer (25 mM Tris pH7.5, 25 mM imidazole, 300 mM sodium chloride) on ice. Freeze at -70°C for 30 min, thaw in running water, and disrupt cells by ultrasonication. The lysed cell solution was centrifuged at 4°C for 30 minutes at high speed (20000 r.p.m.), and then the supernatant was taken a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com