Antibacterial peptide CCM7WC as well as preparation method and application thereof
An antimicrobial peptide and sequence technology, applied in the field of biomedicine, can solve the problems of low antibacterial activity, high cytotoxicity, and poor stability, and achieve the effect of small molecular weight, simple preparation method, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Chemical Synthesis of Antimicrobial Peptide C-CM6 from Transformed Green Sea Turtle
[0020] The green sea turtle antimicrobial peptide Cm-CATH2 is a polypeptide encoded by a gene, containing 33 amino acid residues, a molecular weight of 4089.9Da, and an isoelectric point of 12.96. The full sequence of the green sea turtle antimicrobial peptide Cm-CATH2 is: Arg 1 Arg 2 Ser 3 Arg 4 Phe 5 Gly 6 Arg 7 Phe 8 Phe 9 Lys 10 Lys 11 Val 12 Arg 13 Lys 14 Gln 15 Leu 16 Gly 17 Arg 18 Val 19 Lys 20 Arg 21 His 22 Ser 23 Arg 24 Ile 25 Thr 26 Val 27 Gly 28 Gly 29 Arg 30 met 31 Arg 32 Phe 33 (SEQ ID NO. 1). Because Cm-CATH has the characteristics of large molecular weight, high immunogenicity and toxicity, and poor stability, according to its amino acid sequence, we first shortened it by sequence cutting method to obtain the fragment Arg 1 Arg 2 Ser 3 Arg 4 Phe 5 Gly 6 Arg 7 Phe 8 Phe 9 Lys 10 Lys 11 Val 12...
Embodiment 2
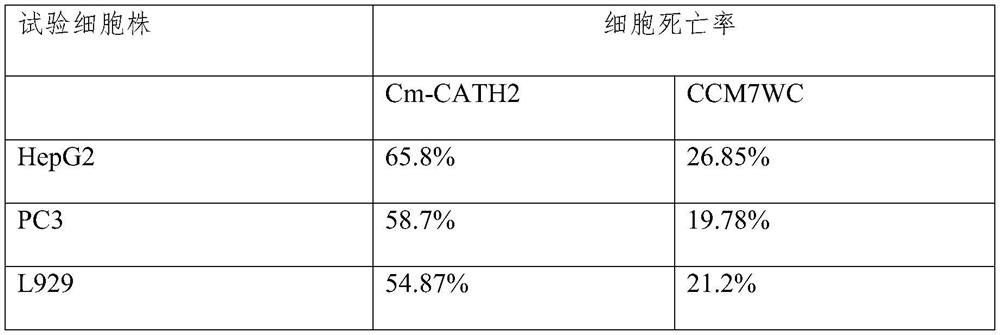
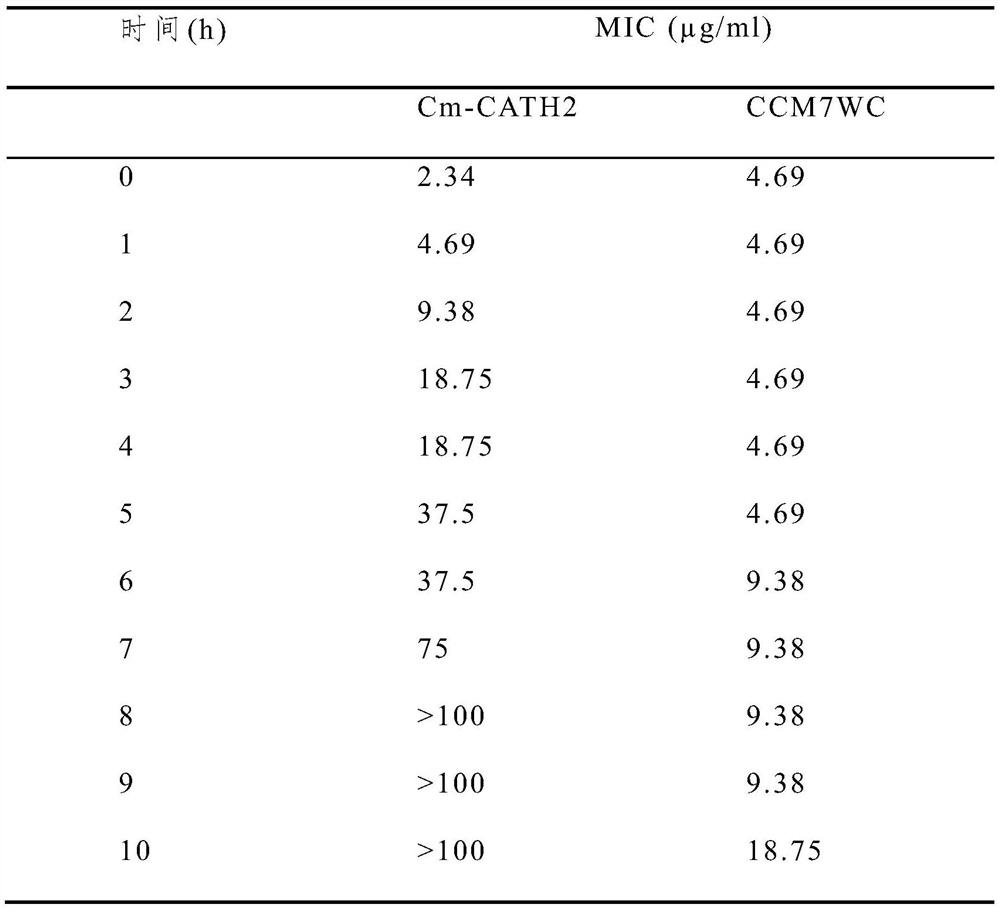
[0027] CCM7WC pharmacological experiment:
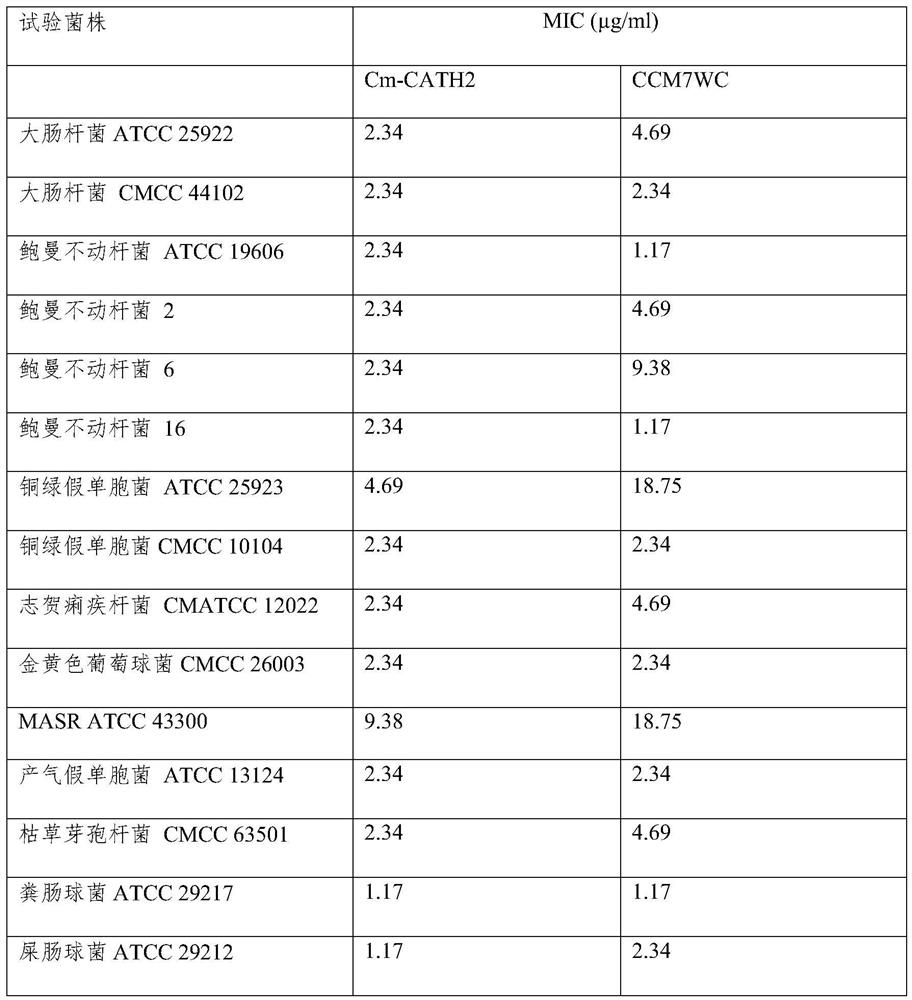
[0028] 1. Determination of antibacterial activity of CCM7WC:
[0029] (1) Pick the test strains preserved on the slant and spread them evenly on the MH solid culture medium (Beijing Suo Laibao Technology Co., Ltd.) plate, place the sterilized 0.5cm diameter filter paper on the surface of the culture medium, Add dropwise 10 μl of 2 mg / ml antimicrobial peptide C-CM6 sample solution dissolved in sterilized deionized water, incubate upside down at 37°C for 18-20 hours, and observe whether the inhibition zone is formed or not. If the sample has antibacterial activity, a clear and transparent bacteriostatic zone will be formed around the filter paper, and the larger the bacteriostatic zone, the stronger the antibacterial activity of the sample.
[0030] (2) Determination of Antimicrobial Peptide CCM7WC Minimum Inhibitory Concentration (2-fold dilution method):
[0031] Select the strains with the inhibition zone in the previous experimen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com