Fluorescent probe for rapidly detecting hydrazine compounds as well as synthesis and application of fluorescent probe
A hydrazine compound and fluorescent probe technology, applied in the field of fluorescent probes, can solve the problems of inability to realize real-time analysis and biological imaging, long detection time, expensive instruments, etc., and achieve fast and efficient detection, low synthesis cost, and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This example provides a method for the synthesis of fluorescent probes for rapid detection of hydrazine compounds, including the preparation of A-1 and A-2, the specific steps are as follows:
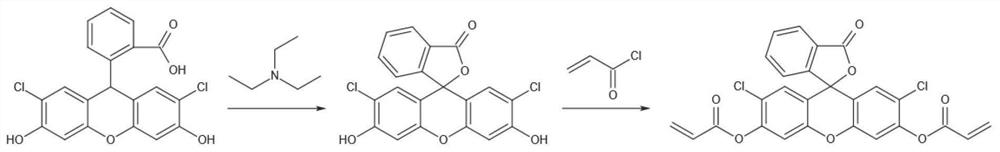
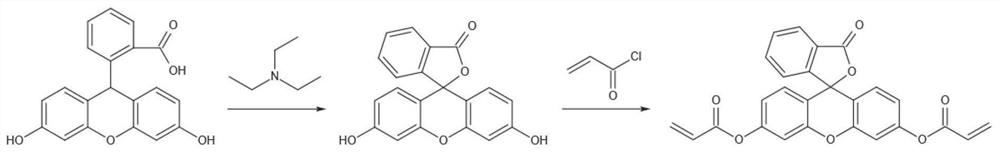
[0040] The method for preparing 0.3 g of probe A-1 includes the following steps: Accurately weigh 0.60 g (1.5 mmol) of dichlorofluorescein and add it to a 100 ml round-bottomed flask, add 20 mL of dichloromethane and stir to dissolve under nitrogen protection, and then dissolve it at 0 ° C. Add 0.20mL triethylamine (1.8mmol) and 0.25mL acryloyl chloride (3mmol) successively under ice bath, mix and stir for 10min, and then react at room temperature for 12h. Remove the solvent, use petroleum ether-ethyl acetate eluent with a volume ratio of 6:1 to elute with a gradient of 25%-65% / 60min, and the flow rate during elution is 6mL / min, and the probe is obtained after column chromatography A-1, the synthetic route of probe A-1 is as follows figure 1 shown.
[0041] The method for prepa...
Embodiment 2
[0044] This example provides the application of a fluorescent probe for the rapid detection of hydrazine compounds, and illustrates that the fluorescent probe synthesized in Example 1 can detect hydrazine compounds with naked eyes.
[0045]Add 10 μL of hydrazine compound (500 μM, 50 eq) into the buffer solution containing the fluorescent probe, the buffer solution is PBS and DMSO solution with a volume ratio of 9:1, the concentration is 10 μM, and the pH is 7.4. After fully reacting for 1 h, under natural light, Compared with the blank solution, the solution system becomes obviously yellow-green after the reaction of the fluorescent probe with the analyte; under the 325nm ultraviolet lamp, it is found that the hydrazine compound shows obvious yellow-green fluorescence, which shows that the fluorescent probe synthesized in Example 1 The probe can realize naked-eye detection of hydrazine compounds.
Embodiment 3
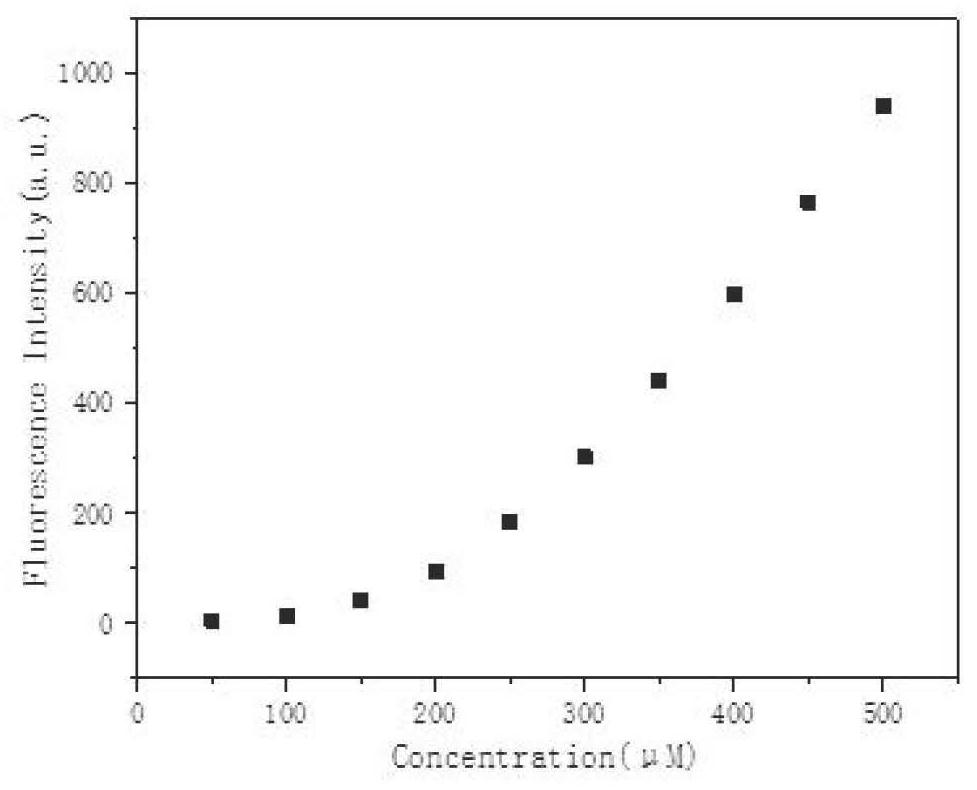
[0047] Such as Figure 2-10 , This example provides an application of a fluorescent probe for rapid detection of hydrazine compounds, indicating that the fluorescence intensity of the fluorescent probe synthesized in Example 1 increases with the increase in the concentration of hydrazine compounds.
[0048] A-1: Add hydrazine, hydrazine sulfate, hydrazine nitrate, partial di methylhydrazine, respectively detect the change of fluorescence intensity at 523nm. The result is as Figure 3-6 As shown, the fluorescence intensity of probe A-1 increases significantly with the increase of the concentration of the analyte, and the calculated detection limits of probe A-1 for hydrazine, hydrazine sulfate, hydrazine nitrate and unsymmetrical dimethylhydrazine are respectively: 0.21 μM (6.7ppb), 0.24μM (31.2ppb), 0.21μM (19.9ppb) and 0.64μM (38.5ppb).
[0049] A-2: Add hydrazine, hydrazine sulfate, hydrazine nitrate, partial di methylhydrazine, respectively detect the change of fluoresc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com