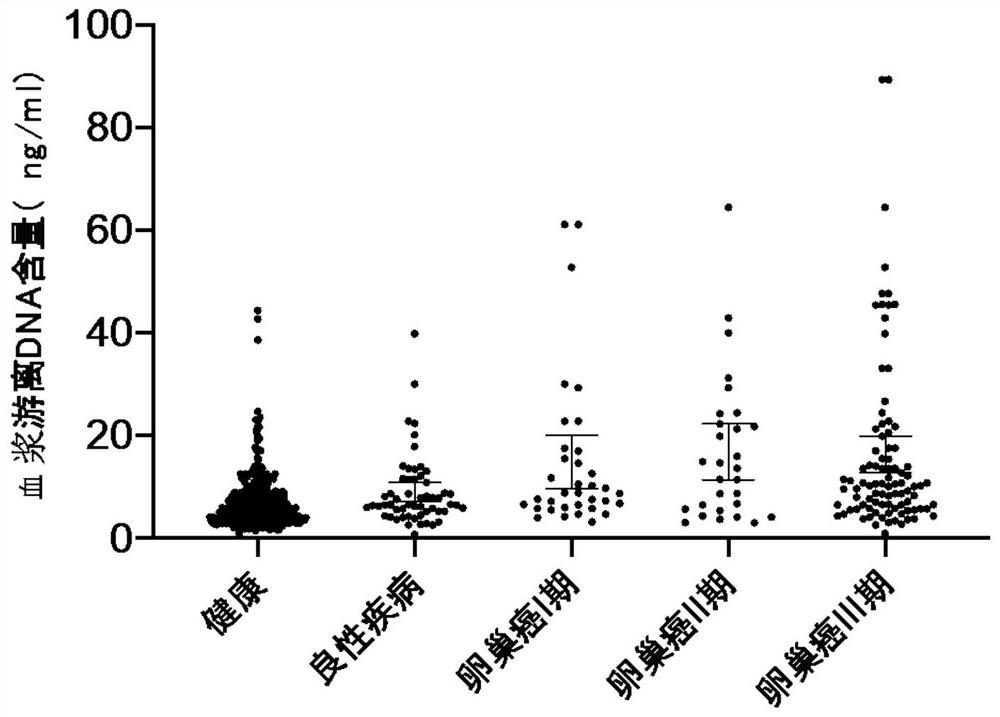
Establishment of composition for early diagnosis of ovarian cancer
A technology for ovarian cancer and in vitro diagnosis, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, instruments, etc., can solve the problems of low tumor burden, no mutation detection or missed detection, and low ctDNA mutation abundance, etc. To achieve the effect of improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] The preparation of the capture antibody can be obtained by linking the monoclonal antibody against the target biomarker to the magnetic particles under appropriate reaction conditions in the presence of a coupling solution. Further, the detection antibody can be a monoclonal antibody labeled with alkaline phosphatase, a monoclonal antibody labeled with alkaline phosphatase, and combinations thereof.
[0098] As an embodiment of the protein chip of the present invention, the capture antibody is immobilized on microspheres carrying specific detectable signals (molecules) to prepare a liquid-phase protein chip, and the principle of detection by using the liquid-phase protein chip is: A single microsphere is passed through a detection channel, and both lasers are used to simultaneously detect the microsphere identification signal and the detectable signal on the microsphere. One type of laser excites the microsphere identification signals on the microspheres. According to t...
Embodiment 1
[0153] 1. Blood Sample Collection
[0154] Venous blood was collected using free DNA blood collection tubes (LBgard blood collection tubes, Biomatirca, USA), and samples were sent to Shanghai Yunsheng Medical Laboratory (Shanghai, China) at room temperature (4°C-37)°C for plasma and white blood blood cell, WBC) separation. All subjects underwent 10 mL whole blood sample collection. Plasma and peripheral blood samples were drawn before anesthesia on the day of surgery. The plasma of healthy volunteers in the biological sample bank is stored at -80°C, not less than 3mL, and the storage time is within 2 years, and it is transported to Shanghai Yunsheng Medical Laboratory by dry ice.
[0155] 2. Extraction of cfDNA and genomic DNA
[0156] Peripheral blood (10 mL for each freshly collected sample, 3 mL for biobank samples) was centrifuged at 4°C, 1600 g, for 10 minutes. Plasma (upper yellow layer) and white blood cells (middle layer) were collected, and then the upper layer of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com