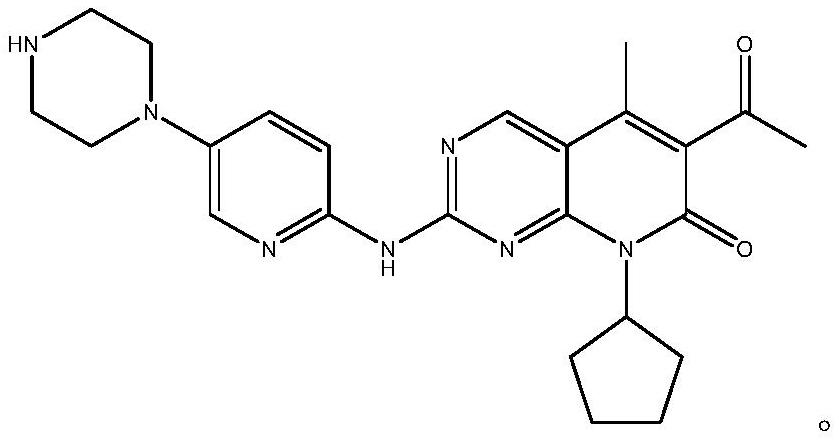
Low-cost preparation method of palbociclib
A low-cost, high-compound technology, applied in the field of low-cost preparation of palbociclib, can solve the problems of rising cost of palbociclib, and achieve the effects of good quality, high yield, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
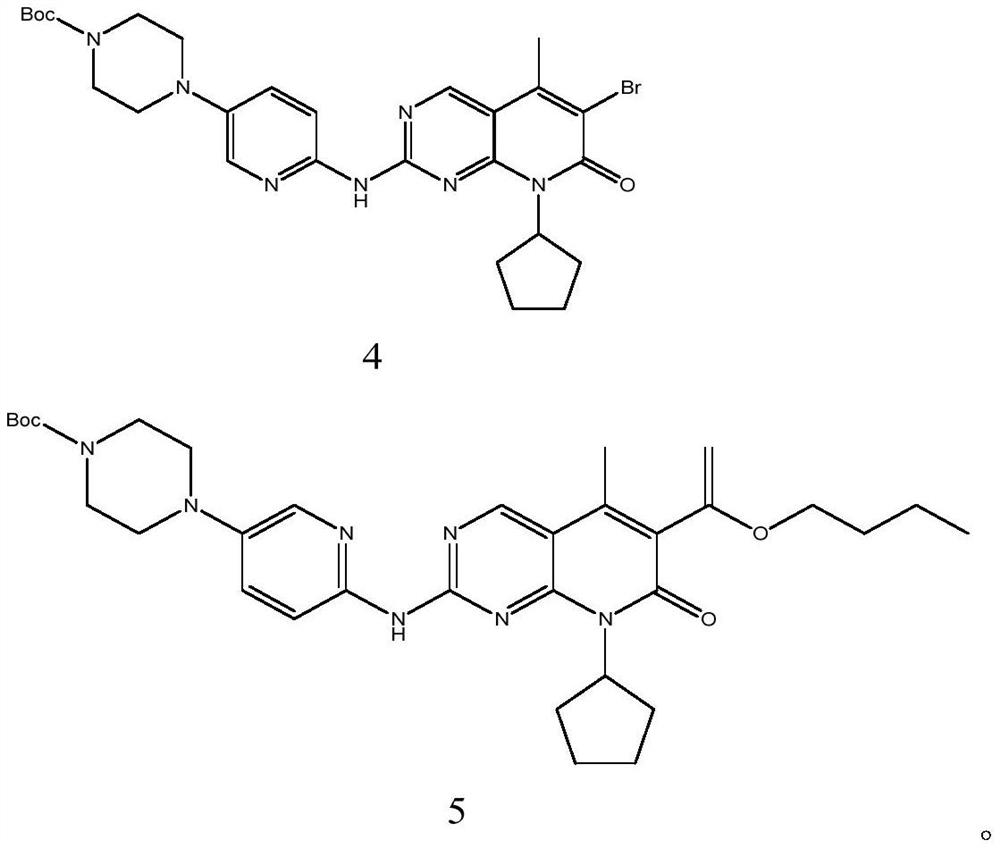
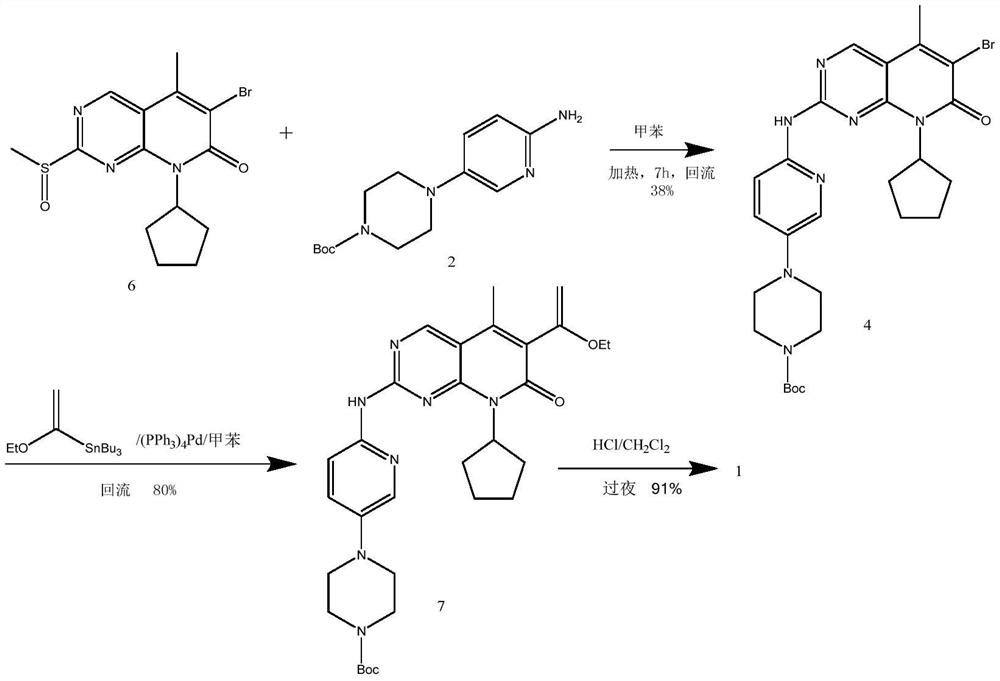
[0066] Example 1: 4-(6-((6-bromo-8-cyclopentyl-7,8-dihydro-5-methyl-7-oxopyrido[2,3-D]pyrimidine-2- Base) amino)-3-pyridyl)-1-piperazinecarboxylate tert-butyl ester (compound of formula 4) laboratory preparation
[0067] In a 5L reaction flask, add 167g (0.6mol) tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate, 1600ml toluene, protect with nitrogen, and add 750ml 1mol / L sodium hexamethyldisilazide tetrahydrofuran solution, keep stirring at 20-30°C for 30 minutes. Disperse 171g (0.5mol) of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one in 600ml of toluene, slowly pour After adding the reaction solution, keep it at 30°C for 4 hours, TLC detects that 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one disappears It is the end point of the reaction (developing solvent: ethyl acetate / n-hexane=1 / 4). After the reaction is complete, slowly add a mixture of 340ml acetone and 170ml water, stir the mixture overnight, gradually pr...
Embodiment 2
[0068] Example 2: 4-(6-((6-bromo-8-cyclopentyl-7,8-dihydro-5-methyl-7-oxopyrido[2,3-D]pyrimidine-2- Base) amino)-3-pyridyl)-1-piperazinecarboxylate tert-butyl ester (compound of formula 4) laboratory preparation
[0069] In a 5L reaction flask, add 167g (0.6mol) tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate, 1600ml toluene, protect with nitrogen, add dropwise 750ml1mol / L tetrahydrofuran solution of lithium hexamethyldisilazide, keep stirring at 20-30°C for 30 minutes. Disperse 171g (0.5mol) of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one in 600ml of toluene, slowly pour After adding the reaction solution, keep it at 30°C for 4 hours, TLC detects that 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one disappears It is the end point of the reaction (developing solvent: ethyl acetate / n-hexane=1 / 4). After the reaction is complete, slowly add a mixture of 340ml acetone and 170ml water, stir the mixture overnight, grad...
Embodiment 3
[0070] Example 3: 4-(6-((6-bromo-8-cyclopentyl-7,8-dihydro-5-methyl-7-oxopyrido[2,3-D]pyrimidine-2- Base) amino)-3-pyridyl)-1-piperazinecarboxylic acid tert-butyl ester (formula 4 compound) industrial preparation
[0071] In a 2000L reactor, add 725kg of toluene, 84kg of tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate, protect it with nitrogen, and add 375L of 1mol / L hexa The tetrahydrofuran solution of sodium methyldisilazide was kept at 20-30° C. and stirred for 30 minutes. Disperse 86kg of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one in 270kg of toluene, slowly pour into the reaction solution, add After completion, keep the reaction at 30°C for 4h, TLC detects that 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one disappears as the end point of the reaction ( Developing solvent: ethyl acetate / n-hexane=1 / 4). After the reaction is complete, slowly add a mixture of 130kg acetone and 85kg water, stir the mixture ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com