Application of sodium phenylbutyrate and metabolites thereof in preparation of medicine for preventing or treating chemotherapy-induced peripheral neuropathic pain
A metabolite, sodium phenylbutyrate technology, applied in the field of medicine to achieve the effect of increasing the threshold of mechanical pain paw withdrawal, significant analgesic effect, and improving demyelination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The effect of embodiment 1 sodium phenylbutyrate on general state of mice
[0033] 1.1 Modeling
[0034] Female C57BL / 6 mice were injected intraperitoneally with paclitaxel, 4.5mg / kg, once every other day, for a total of 4 injections (1, 3, 5, 7d) to establish chemotherapy-induced peripheral neuralgia (CIPN) in mice Model, normal control group animals were injected with normal saline.
[0035] 1.2 Experimental design
[0036] Group design: normal control group, model control group, ZK034 low-dose group, ZK034 middle-dose group, ZK034 high-dose group and pregabalin group;
[0037] See the table below for specific grouping information:
[0038]
[0039]
[0040] Note: The first digit of the animal number represents the group (1, 2, 3, 4, 5 and 6 represent the normal control group, model control group, ZK034 low-dose group, ZK034 middle-dose group, ZK034 high-dose group and pregabalin respectively Group). The second letter represents gender (F is female), the la...
Embodiment 2
[0055] The effect of embodiment 2 sodium phenylbutyrate on mouse body weight
[0056] 2.1 Modeling is the same as in Example 1
[0057] 2.2 The experimental design is the same as in Example 1.
[0058] 2.3 Administration information is the same as that in Example 1.
[0059] 2.4 Body weight determination
[0060] Measurement time: 2 times a week;
[0061] Measuring animals: all surviving experimental animals planned to be measured.
[0062] 2.5 Statistics are the same as in Example 1.
[0063] 2.6 Test results
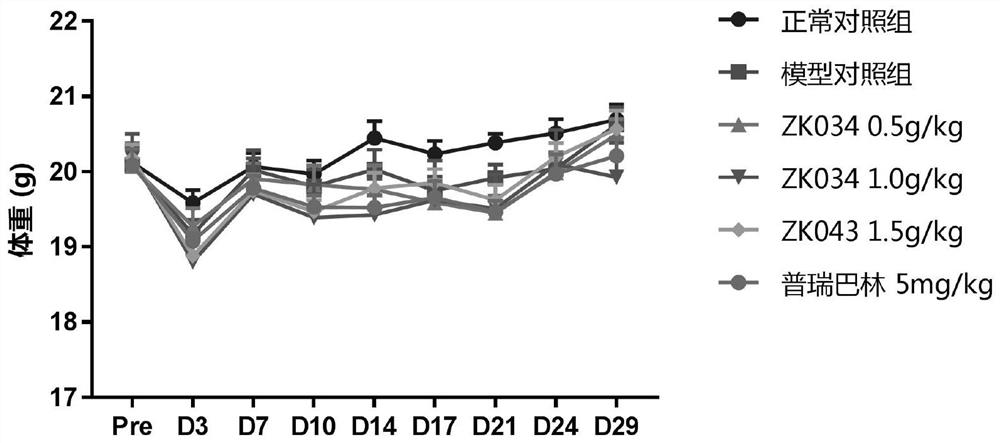
[0064] Throughout the test ( figure 1 , Table 1), the weight of the animals in the normal control group was increasing, and the overall change trend of the body weight of the animals in the other groups was the same. After the drug intervention, there was no significant effect on the change of the body weight of the animals. in figure 1 The data in the table are expressed as mean ± standard error (Mean ± SEM), and each test group uses 10 animals for statistical...
Embodiment 3
[0068] Example 3 Effect of sodium phenylbutyrate on the paw withdrawal threshold of mice with mechanical pain
[0069] 3.1 Modeling is the same as in Example 1
[0070] 3.2 The experimental design is the same as in Example 1.
[0071] 3.3 Administration information is the same as that in Example 1.
[0072] 3.4 Determination of mechanical pain withdrawal threshold (MWT)
[0073] Measurement time: 1 test before modeling, 7d, 14d, 20d, 25d, 30d after modeling;
[0074] Measuring animals: all surviving experimental animals that are planned to be measured;
[0075] Measuring method: Before testing, the animals were first put into the testing box to adapt for 3 consecutive days, 30 minutes per day; each time the animal was put into the testing box to adapt to 10 minutes before testing, each animal was tested 3 times in a row to get the average value, and each interval 5min.
[0076] 3.5 Statistics are the same as in Example 1.
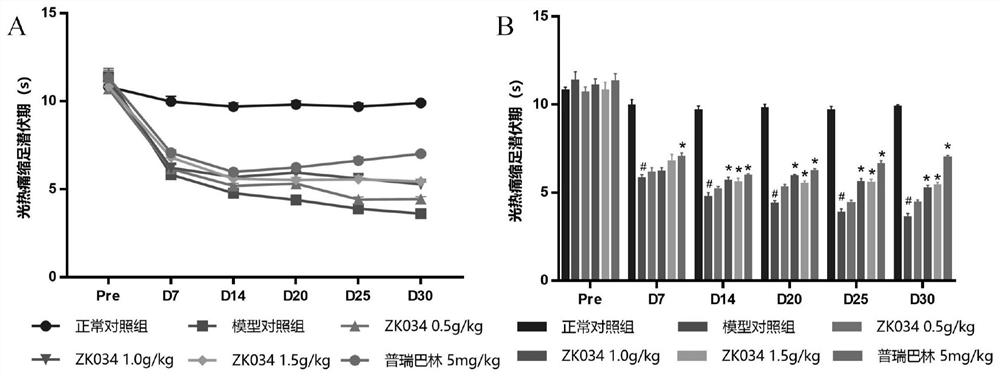
[0077] 3.6 Test results
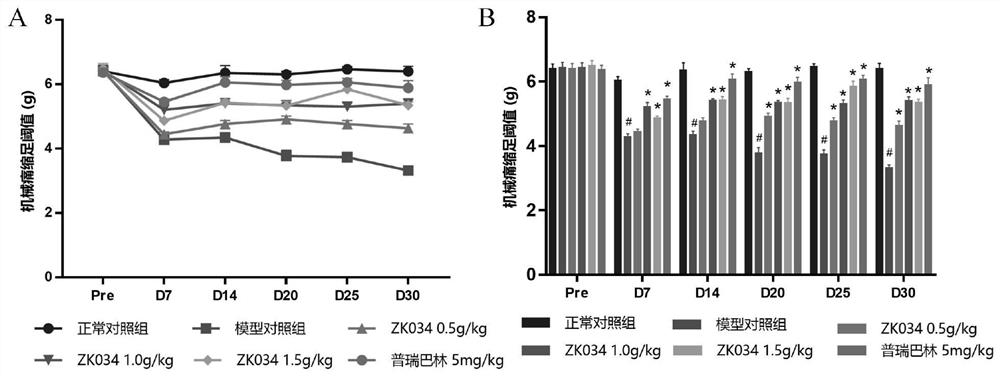
[0078] The average m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com