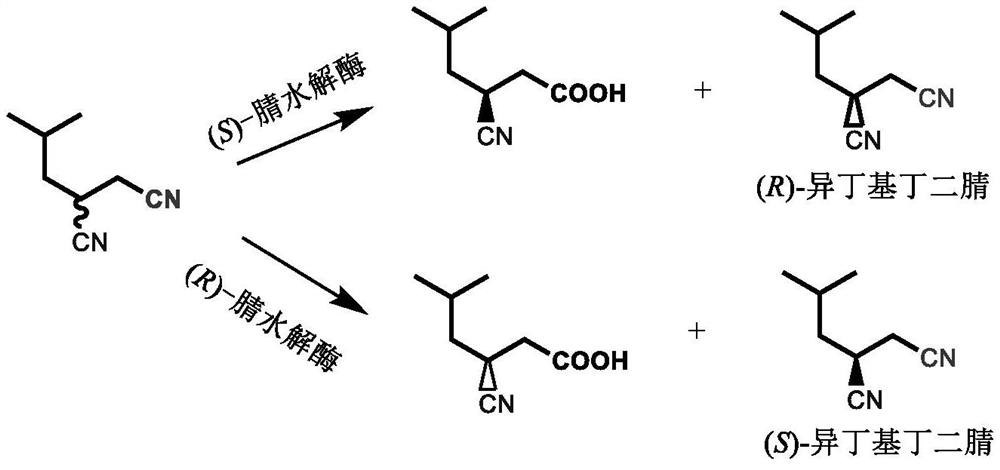
Racemization method of optically pure isobutyl butanedinitrile
A technology of pure isobutylsuccinonitrile and isobutylsuccinonitrile, applied in chemical instruments and methods, chemical recovery, organic chemistry, etc., can solve the problems of difficult catalyst recovery, a large amount of waste acid, waste water, etc., and achieve the goal of eliminating Fast rotation speed and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
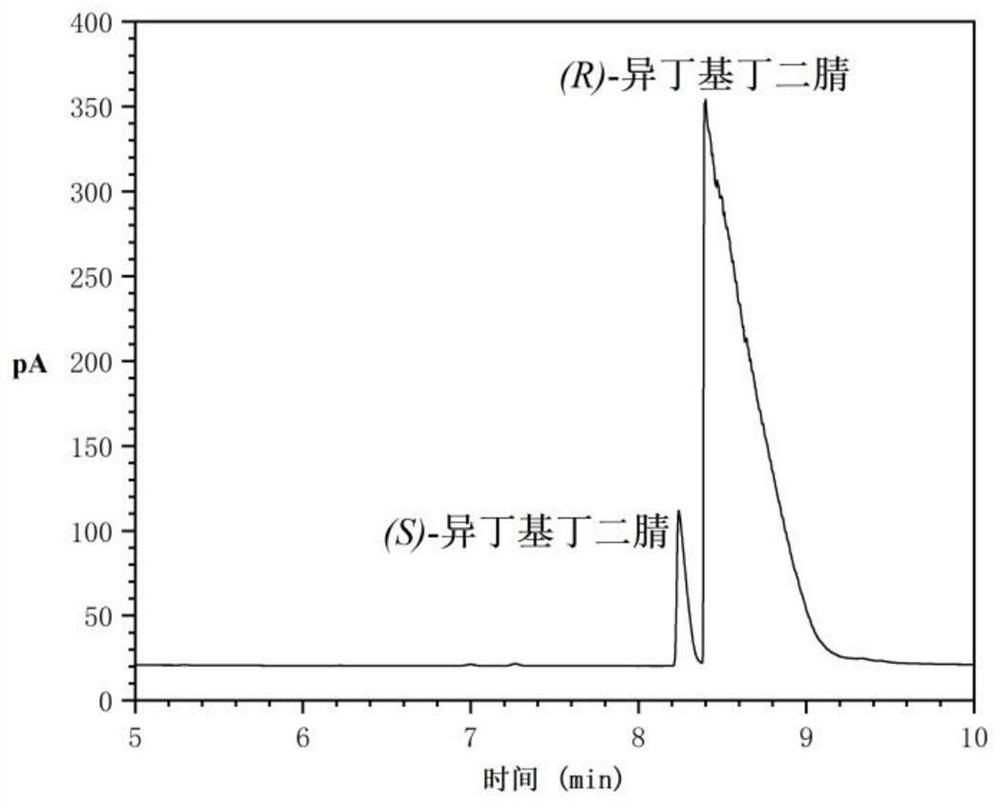
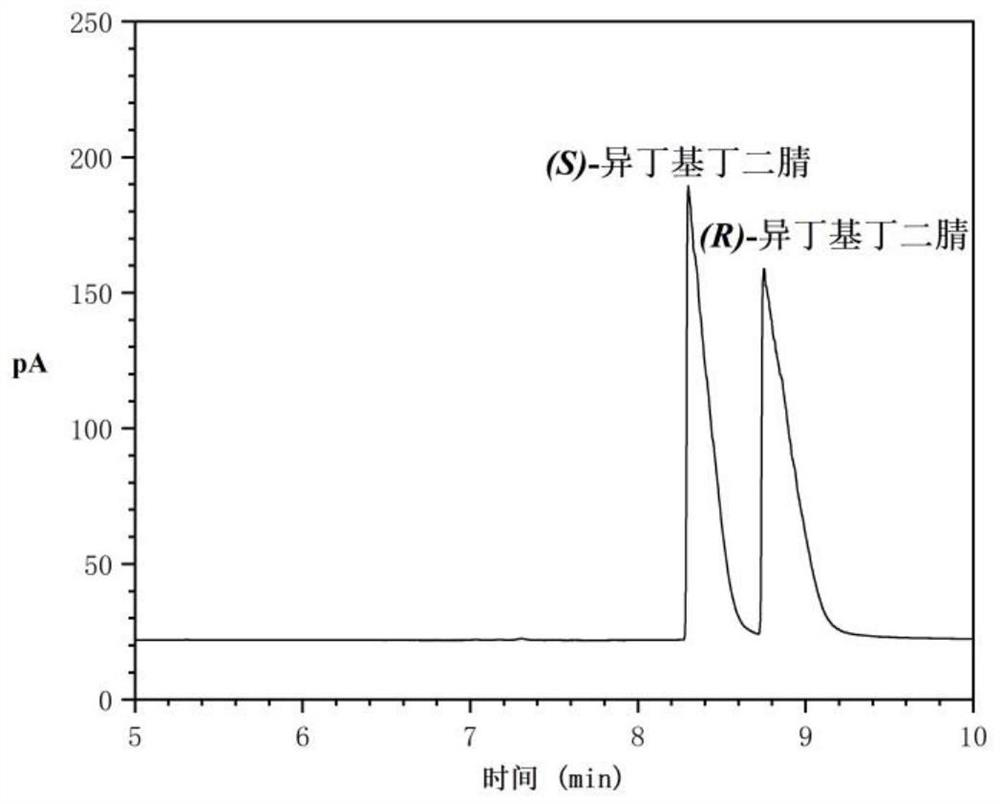
[0024] In a 25mL reactor, add 0.5g (R)-isobutylsuccinonitrile (ee value 89.01%, see HPLC figure 1 ) and 10mL ethanol, after incubation for 30min, add 0.5g solid base catalyst HND-63, react at 35°C and 150rpm for 1h, centrifuge the reaction solution at 8000rpm for 10min, and wash the centrifuged HND-63 with ethanol. Combine the supernatant and washing liquid after centrifugation, and rotate at 70°C for 2h to obtain 0.45g racemic isobutylsuccinonitrile (ee value 1.78%, yield 90%, see HPLC figure 2 ).
[0025] The gas chromatographic column model is BGB-174, and the chromatographic conditions are: the injection volume is 1.0 μL, the temperature of the injection port and the detector is 250 °C, the column temperature is 120 °C, keep for 15 min, and then raise the temperature to 170 °C at 10 °C / min. The flow rate was 1.0 mL / min.
[0026] The enantiomeric excess value (ee value) is calculated with reference to the calculation method of Rakels et al. (Enzyme & Microbial Technology...
Embodiment 2
[0028] Add 0.5g (R)-isobutylsuccinonitrile (ee value 89.01%) and 10mL ethanol into a 25mL reactor, keep warm for 30min, add 0.5g solid base catalyst HND-63, and react at 40°C and 150rpm After 10 hours, the reaction solution was centrifuged at 8000rpm for 10 minutes, the HND-63 obtained by centrifugation was washed with ethanol, the supernatant and washing solution after centrifugation were combined, and rotary steamed at 70°C for 2 hours to obtain 0.45 g of racemic isobutylbutyl Dinitrile (ee value 1.08%, yield 90%).
Embodiment 3
[0030] In a 25mL reactor, add 0.5g (R)-isobutylsuccinonitrile (ee value 89.01%) and 10mL ethanol, after 30min of heat preservation, add 0.5g solid base catalyst HND-63, at 20°C, 150rpm After reacting for 1.5h, the reaction solution was centrifuged at 8000rpm for 10min, the HND-63 obtained by centrifugation was washed with ethanol, the supernatant after centrifugation was combined with the washing solution, and the solution was rotary evaporated at 70°C for 2h to obtain 0.47g of racemic isobutyl Succinonitrile (ee value 0.68%, yield 94%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com