Recombinant phospholipase D mutant and application thereof in synthesis of phosphatidylserine
A technology of phosphatidylserine and mutants, applied in the field of bioengineering, can solve the problems of reducing PS yield, increasing cost, and failure to successfully transform lecithin, etc., and achieve the goal of accelerating the industrialization process, improving production capacity, and high transphosphatidyl activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
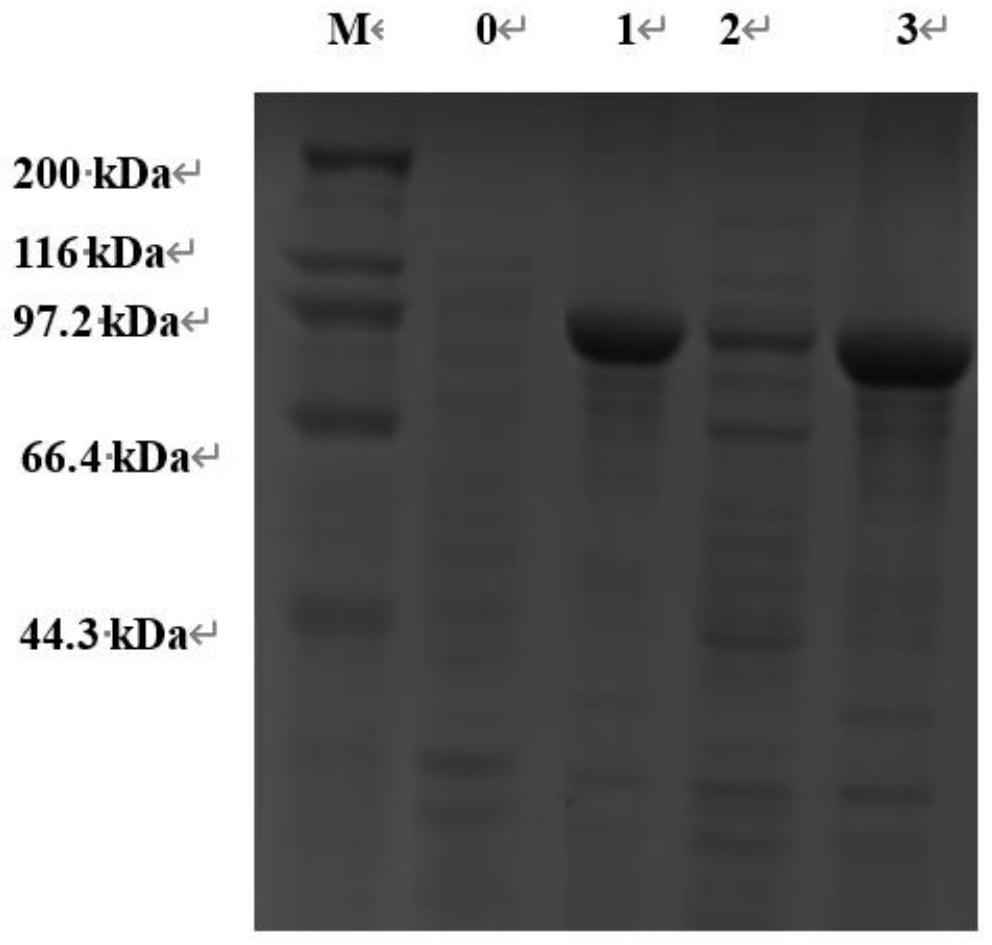
[0067] Embodiment 1: Expression and purification of SrPLD
[0068] Construction of genetically engineered bacteria and protein expression:
[0069] Taking the nucleotide sequence (shown in SEQ ID NO.2) of the target protein coding gene in Streptomyces racemochromogenes as template, using F1 and R1 as primers (the underlines are respectively BamH I and EcoR I restriction enzyme cut sites Point) PCR amplification, the amplification conditions are: 95°C for 5min, 29 cycles (98°C for 10s, 55°C for 15s, 72°C for 2min), 72°C for 5min.
[0070] F1: aaatgggtcgc ggatcc ttggcacgcacc;
[0071] R1: gacggagctc gaattc tcaggcctggcaga.
[0072] Obtain the cDNA sequence of the coding region of the PLD gene, recover the PCR product and connect it with the pET-28a(+) plasmid vector that has undergone the same double digestion through homologous recombination to obtain the recombinant expression plasmid pET-28a(+)-PLD, and convert the recombinant plasmid pET-28a(+) to -28a(+)-PLD was trans...
Embodiment 2
[0075] Example 2: Recombinant bacteria E.coli BL21 / pET-28a(+)-Sr MBP Construction of PLD and Phospholipase D Sr MBP Expression of PLD
[0076] (1) Recombinant bacteria E.coli BL21 / pET-28a(+)-Sr MBP Construction of PLDs
[0077] In order to solve the problem of heterologous expression of inclusion bodies in SrPLD, our research group found that SrPLD contains a signal peptide sequence (the nucleic acid sequence of the signal peptide is shown in SEQ ID NO.6), so a recombinant plasmid that removes the signal peptide should be constructed. Using the recombinant plasmid pET-28a(+)-PLD prepared in Example 1 as a template, using F2 and R2 as primers (the underlines are respectively BamH I and EcoR I restriction enzyme sites) PCR amplification, the amplification conditions are: : 95°C for 5min, 29 cycles (98°C for 10s, 55°C for 15s, 72°C for 2min), 72°C for 5min.
[0078] F2: atgggtcgc ggatcc gcttcgccga;
[0079] R2: gacggagctc gaattc tcacgcttggcac.
[0080] After the PCR pro...
Embodiment 3
[0090] Example 3: Preparation of Phosphatidylserine by Whole Cells
[0091] Add 0.08g of whole cells E.coli BL21 / pET-28a(+)-Sr expressing PLD protein after induction culture to 10mL small brown bottle MBP PLD or E.coli BL21 / pET-28a(+)-PLD, 0.24g L-serine, 0.3g phosphatidylcholine (PC), 1mL acetic acid-sodium acetate buffer pH6.0, 3mL ethyl acetate, in React at 40° C. and 400 rpm in a constant temperature shaker for 12 hours, and the reaction pH is 6.0. The converted reaction solution sample was centrifuged at 12000rpm for 5-10min, the upper organic phase was absorbed, and the organic phase was volatilized and then dissolved with the mobile phase (n-hexane / isopropanol / acetic acid=8 / 8 / 1), passed through 0.22μm After the organic membrane, high-phase liquid chromatography (HPLC) analysis was performed.
[0092] The obtained E.coli BL21 / pET-28a(+)-Sr MBP PLD and E.coli BL21 / pET-28a(+)-PLD were used to prepare phosphatidylserine in whole cells, and the results were as follows: us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com