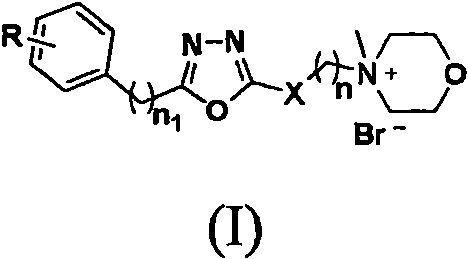
1, 3, 4-oxadiazole compound containing morpholine group as well as preparation method and application thereof
A technology of oxadiazoles and compounds, applied in the field of medicinal chemistry, can solve the problems of high phytotoxicity of rice leaves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Preparation of intermediate 2-((8-bromooctyl)thiol)-5-phenyl-1,3,4-oxadiazole
[0048] 5-phenyl-1,3,4-oxadiazole-2-thiol (1mmol), K 2 CO 3 (1.3mmol) and 8mL DMF were added into a 25mL round bottom flask, then 1,8-dibromooctane (1.3mmol) was added and stirred at room temperature for 2 hours to end the reaction. Precipitation, column chromatography (eluent petroleum ether [ethyl acetate = 10 [1, V / V]) to obtain the intermediate. At the same time, for intermediates with other chain lengths, except that 1,8-dibromooctane was replaced with different chain lengths, the experimental steps and feeding ratio of other intermediates were consistent with those in Example 1.
Embodiment 2
[0049] Example 2: 4-methyl-4-(8-((5-phenyl-2-1,3,4-oxadiazolyl)thiol)octyl)morpholin-4-ium bromide
[0050] 2-((8-Bromooctyl)thiol)-5-phenyl-1,3,4-oxadiazole (0.4 mmol) and 4-methylmorpholine (4.55 mmol) were dissolved in 4 mL of CH 3 CN and put it into a 15mL reaction flask, and reflux at 85°C for 8h. TLC followed the completion of the reaction. Water (3 x 20 mL) and ethyl acetate (50 mL) were added for extraction. The organic phase was anhydrous Na 2 SO 4 Dry, filter with suction, and concentrate under reduced pressure. The crude residue was further purified by silica gel column chromatography using CH 2 Cl 2 and CH 3 OH (20:1, v / V) was used as the eluent to obtain a light yellow solid with a yield of 63.2%. The rest of the experimental steps and feeding ratios were consistent with those in Example 2.
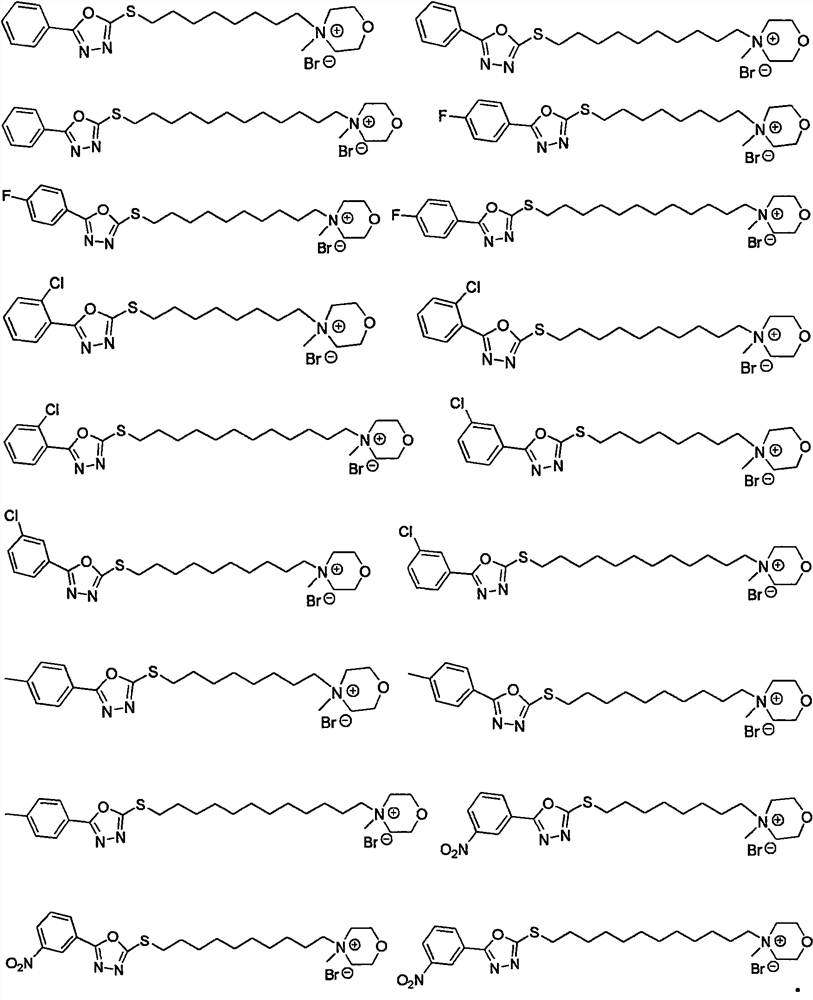
[0051] The remaining 1,3,4-oxadiazole compounds containing morpholine groups were synthesized according to the steps of Examples 1 and 2 using corresponding raw mater...
Embodiment 3
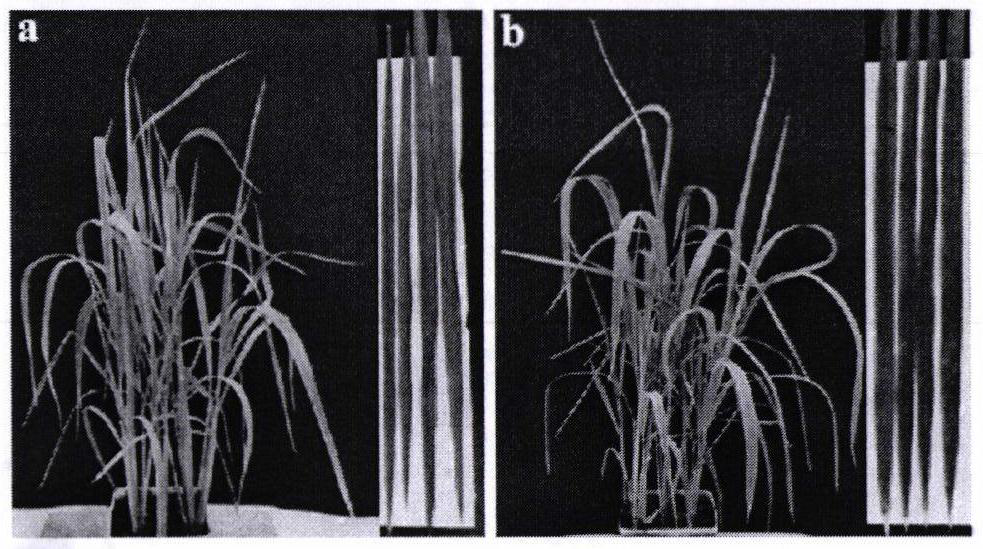
[0076] Based on compound 12 showing the best activity against rice bacterial blight (EC 50 1.40 μg / mL), the in vivo pot experiment of compound 12 on rice bacterial blight was carried out. The specific experimental steps are as follows:
[0077] Protective activity: Compound 12 and the control drug chlorfenadin (20% content preparation) were formulated into a 200 μg / mL drug-containing solution with less than 1% Tween20 solution, and two more compound 12 solutions with a concentration of 200 μg / mL were prepared; Spray the prepared medicinal solution on the surface of rice leaves that have been grown for 8 weeks until there are drops; 595 = 0.6-0.8 The scissors of rice bacterial blight bacteria in the range of 0.8 cut off the tip of the leaf, and soak the wound in the bacterial solution for about 10 seconds. The equal amount of DMSO and the bacterial leaf without adding the drug are set as a control. Each treatment has three After 14 days, check the disease situation, record th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com