Synthesis and anticancer activity of conjugated phospholipid compounds
A technology of compounds and conjugates, applied in the field of phospholipid compounds, can solve the problems of toxic and side effects, the effect of limiting the use of chemotherapeutic agents, and the decline of leukocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Synthesis of rac-1,3-O,O-benzylidene glycerol (1)
[0013] 1mol glycerin and 1.2mol benzaldehyde were reacted at 140-170°C for 3h, distilled under reduced pressure, collected fractions at 180-182°C / 1.1Pa, diluted to 200-300ml by adding ether, and washed successively with 10% K 2 CO 3 and water, and the organic phase was washed with anhydrous K 2 CO 3 Dry, remove the solvent, dilute the residue with a mixture of petroleum ether and benzene, cool to -20°C, filter the precipitated solid, cool the filtrate to -20°C, combine the solids obtained twice and recrystallize with ether, the yield 74%, m.p.80.0-84.0°C.
[0014] Synthesis of rac-2-O-hexadecyl-1,3-O,O-benzylidene glycerol (2)
[0015] Add 2.1 mmol of sodium hydride (80%) to 20 mmol THF cooled to 0°C, add dropwise a solution of 20 mmol of compound (1) dissolved in 30 mL of THF under cooling in an ice-water bath, react at room temperature for 30 min under stirring, and then add dropwise 21 mmol of Hexadecane bromid...
Embodiment 2
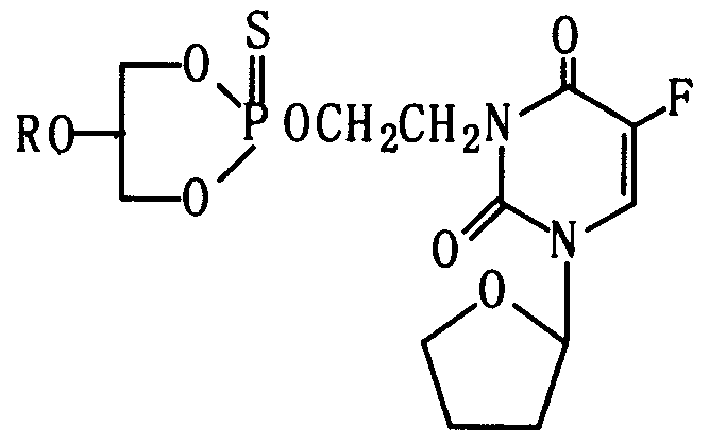
[0022] 2-(2'-Tegafur-N 3 -) Synthesis of ethoxy-2-thio-5-octadecyloxy-1,3,2-dioxaphosphorinane (5b)
[0023] The preparation method is the same as the synthesis of Example 1 (5a), colorless viscous liquid, R f =0.65(G 254 Silica gel plate, the developer is ethyl acetate-petroleum ether v / v=1:1), the yield is 40%. C 31 h 54 FN 2 o 7 Elemental analysis of PS: C, 57.40 (57.41); H, 8.72 (8.36); N, 4.32 (4.32). IR(Film)(cm -1 ): 2908, 1715, 1674, 1464, 1262, 1073, 1022, 839, 767, 716, 638. 1 H NMR: (200Mhz, CDCl 3 , δ, ppm) 0.85 (t, 3H, CH 3 ), 1.22 (sb, 30H, 15CH 2 ), 1.52 (m, 2H, CH 2 -C-O), 1.68-2.46 (m, 4H, 2', 3'-2CH 2 ), 3.52(t, 2H, CH 2 N), 3.55-4.33 (m, 11H, 5CH 2 O, OCH), 5.96(m, 1H, 1'-H), 7.35(d, 1H, 6-H, 2 J F-H = 6.2Hz), 31 P NMR: 63.27 63.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com