Crystal form of Crisabole solvate and preparation method and application of crystal form
A solvate, solvation technology, applied in organic chemistry methods, chemical instruments and methods, active ingredients of boron compounds, etc., can solve problems such as water solubility and low stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Take about 90mg of Crisaborole crystal form, add 1.5mg of pyridine, heat up to 60°C to dissolve to obtain solution 1; take about 6mg of polyethylene glycol, add 4.5mL of water to dissolve to obtain solution 2; add solution 2 dropwise to solution 1 , precipitated solid, continued to stir overnight, and centrifuged to obtain FB-2.
[0094] The samples of Example 1 were taken for characterization, as follows.
[0095] 1. XRPD spectrum analysis
[0096] figure 1 is the XRPD pattern of FB-2, Table 1 shows the X-ray powder diffraction data of FB-2.
[0097] Table 1: X-ray powder diffraction data for FB-2
[0098]
[0099]
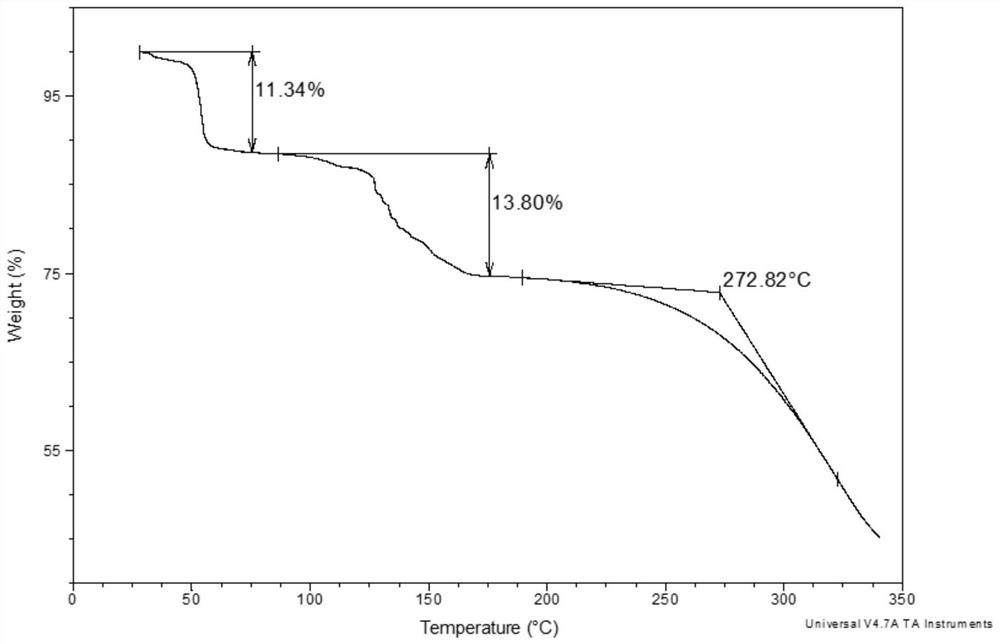
[0100] 2. TGA spectrum analysis
[0101] TGA (thermogravimetric analysis) results such as figure 2 shown. It has a weight loss step before 75°C, the weight loss rate is 11.3%, and its decomposition temperature is 273°C.
[0102] 3. DSC spectrum analysis
[0103] The results of differential calorimetry scanning analysis were as follows: ima...
Embodiment 2
[0111] Take about 30mg of Crisaborole, add 0.5mL of pyridine, heat up to 60°C to dissolve, add dropwise 1.5mL of water, precipitate a solid, and continue stirring overnight to obtain FB-2.
Embodiment 3
[0113] Take about 90mg of Crisaborole, add 0.8mL of pyridine and 2mL of water, dissolve at 60°C and filter, keep the filtrate for 20min, then place the filtrate at 4°C and stir overnight, and the solid precipitates to obtain FB-2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com