Veirofenib camphor sulfonate and preparation method thereof
A technology of camphorsulfonate and vemurafenib, which is applied in the field of medicinal chemistry, can solve the problems of low solubility and oral bioavailability of vemurafenib, serious adverse reactions and the like, and achieves easy control of the crystallization process and good reproducibility. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 42.5 mg of vemurafenib and 20.2 mg of DL-camphorsulfonic acid, add 1 mL of n-heptane and 10 μL of methanol to obtain a suspension, stir the suspension at room temperature for 48 h, filter, and obtain a white solid at room temperature Vacuum-dried for 12 hours to obtain vemurafenib DL-camphorsulfonate.
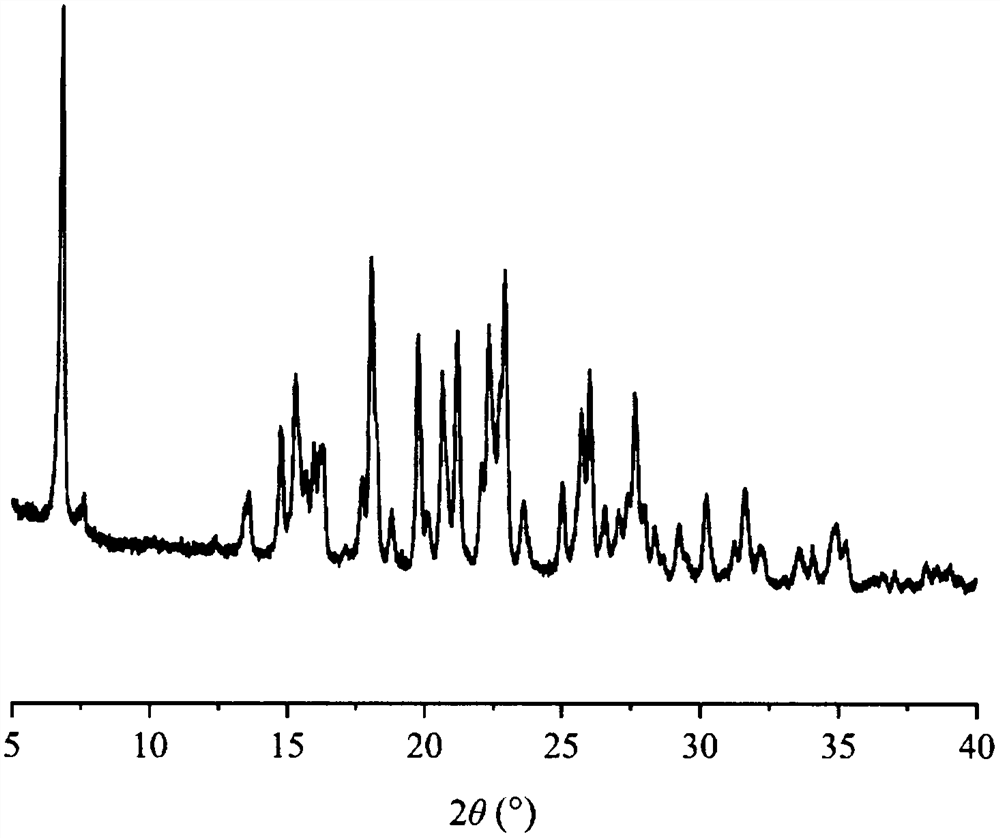
[0041] Carry out X-ray powder diffraction analysis to the vemurafenib DL-camsylate that embodiment 1 makes, its analysis result sees attached figure 1 The X-ray powder diffraction pattern, X-ray powder diffraction data are shown in table 1.
[0042] The X-ray powder diffraction data of the vemurafenib DL-camsylate of table 1 embodiment 1
[0043]
[0044]
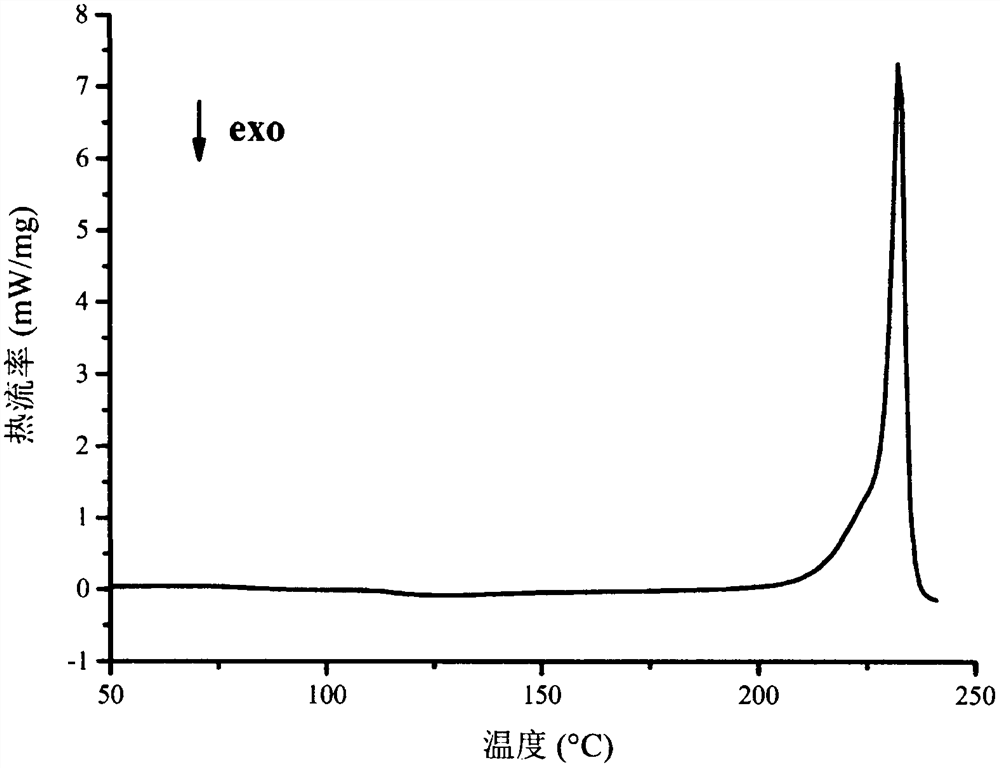
[0045] Differential scanning calorimetry chart as figure 2 As shown, the product presents an endothermic peak at 232.0°C.
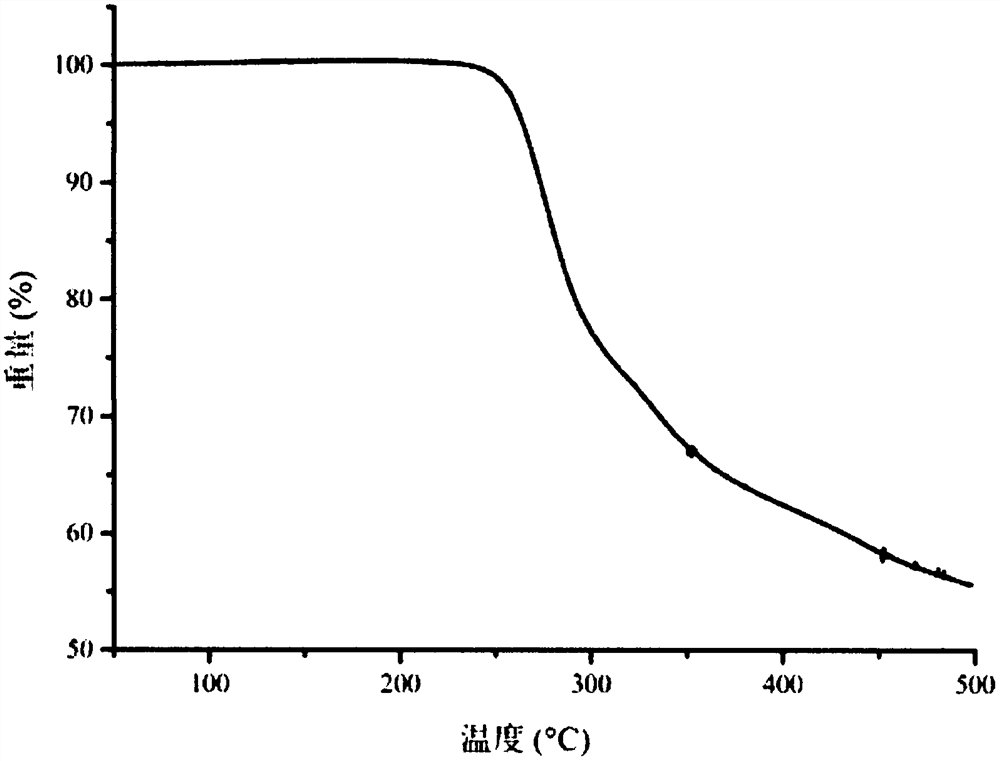
[0046] Thermogravimetric analysis chart such as image 3 shown.
[0047]The positions of the Fourier transform infrared characteristic peaks are: 3432, 3130, 3020, 1742, 1640, 1...
Embodiment 2
[0050] Weigh 45.2 mg of vemurafenib and 20.2 mg of DL-camphorsulfonic acid, add 1 mL of n-heptane and 10 μL of ethanol to obtain a suspension, stir the suspension at room temperature for 48 h, filter, and obtain a white solid at room temperature Vacuum-dried for 12 hours to obtain vemurafenib DL-camphorsulfonate.
[0051] The X-ray powder diffraction data of vemurafenib DL-camsylate prepared in Example 2 are shown in Table 2.
[0052] The X-ray powder diffraction data of the vemurafenib DL-camsylate of table 2 embodiment 2
[0053]
[0054]
Embodiment 3
[0056] Weigh 45.2 mg of vemurafenib and 20.2 mg of DL-camphorsulfonic acid, add 1 mL of n-heptane and 10 μL of acetonitrile to obtain a suspension, stir the suspension at room temperature for 48 h, filter, and obtain a white solid at room temperature Vacuum-dried for 12 hours to obtain vemurafenib DL-camphorsulfonate.
[0057] The X-ray powder diffraction data of vemurafenib DL-camphorsulfonate prepared in Example 3 are shown in Table 3.
[0058] The X-ray powder diffraction data of the vemurafenib DL-camsylate of table 3 embodiment 3
[0059]
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com