Biological preparation for regulating ovarian function as well as preparation method and application of biological preparation
A biological preparation and ovarian function technology, applied in the field of gene recombination, can solve the problems of no treatment or health care system, anti-Müllerian hormone yolk antibody, and inability to take oral administration, etc., to achieve targeted treatment and health care, and solve High cost problem, the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of recombinant anti-Müllerian hormone
[0035] (1) Acquisition of the gene sequence of recombinant anti-Müllerian hormone:
[0036] Referring to the GENBANK accession number NM_000479.5, the amino acid sequence of human anti-Mullerian hormone was taken, and the TAT transduction sequence was connected to the N-terminal, and the reverse translation was the target gene. The target gene sequence was sent to Huada Gene Synthesis to obtain the pYES2 / CT plasmid containing the target gene, and the sequence was qualified, and the target gene sequence as shown in SEQ ID NO:1 was obtained.
[0037] (2) Construction of engineering bacteria:
[0038] The plasmid was introduced into Invscv1 Saccharomyces cerevisiae by the PEG / LiCl method, spread on the ampicillin SD-Ura plate, and incubated at 30°C for 72 hours, and the colony grown on the SD-Ura plate was picked for preservation and sent to Huada Gene for sequencing The target gene was identified, and the se...
Embodiment 2
[0042] Example 2: PEGylation of recombinant anti-Müllerian hormone
[0043] Vacuum freeze-dry the purified target protein AMH to obtain a powder.
[0044] Prepare the reaction buffer system, the buffer system can choose acetate buffer solution with pH 4.0, 5.0, and phosphate buffer solution with pH 6.0, 7.0, 8.0.
[0045] Take the lyophilized AMH and mPEG-SPA in a molar ratio of 1:2 to 1:12, and stir in the reaction buffer system for 2h to 12h at room temperature in the dark using a magnetic stirrer. Dialyzed in 20 volumes of PBS buffer for 12 hours. 0.22 μm sterile filtration treatment, the processed sample is the PEGylated target protein, which is denoted as mPEG-AMH.
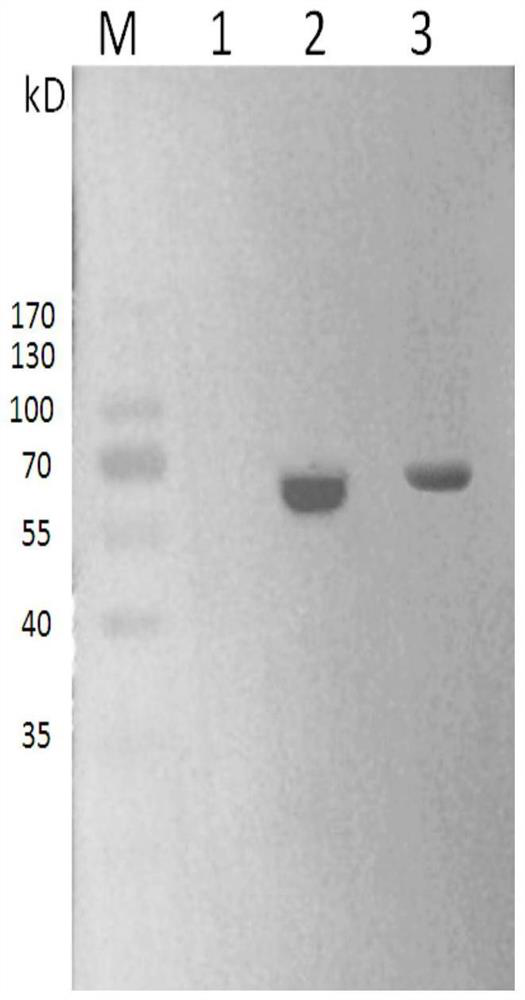
[0046] Adjust the concentration of AMH and mPEG-AMH to 1 mg / mL, use abcam company mouse anti-AMH as the primary antibody (Cat. No. ab239491), and use abcam company goat anti-mouse IgG (HRP) as the secondary antibody (Cat. No. ab6789) for WB experiment verification. The result is as figure 1 As shown, there ...
Embodiment 3
[0047] Example 3: Preparation of recombinant follicle stimulating hormone
[0048] (1) Acquisition of the gene sequence of recombinant follicle stimulating hormone:
[0049] Referring to the amino acid sequences of GENBANK accession number AAA52476.1 and GENBANK accession number ACM91588.1, a flexible linker is used in the middle, and a TAT transduction sequence is connected at the N-terminal, and the reverse translation is the target gene. The target gene sequence was sent to Huada Gene Synthesis to obtain the pYES2 / CT plasmid containing the target gene, and the sequence was qualified, and the target gene sequence as shown in SEQ ID NO:2 was obtained.
[0050] (2) Construction of engineering bacteria:
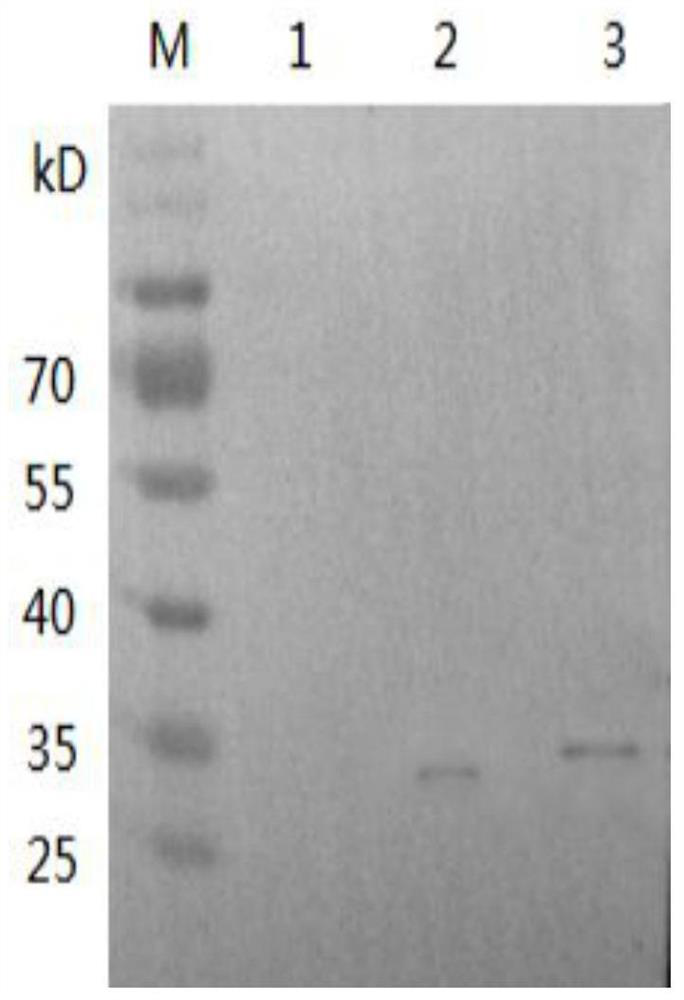
[0051] The plasmid was introduced into Invscv1 Saccharomyces cerevisiae by the PEG / LiCl method, spread on the ampicillin SD-Ura plate, and cultured at a constant temperature of 30°C for 72 hours, and the colony grown on the SD-Ura plate was picked for preservation and sent to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com