Construction and application of engineering strain for biosynthesizing momordica grosvenori glucoside V by taking mogrol as substrate
A technology of mogroside and mogroside, which is applied in the biological field to achieve the effects of high synthetic yield, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
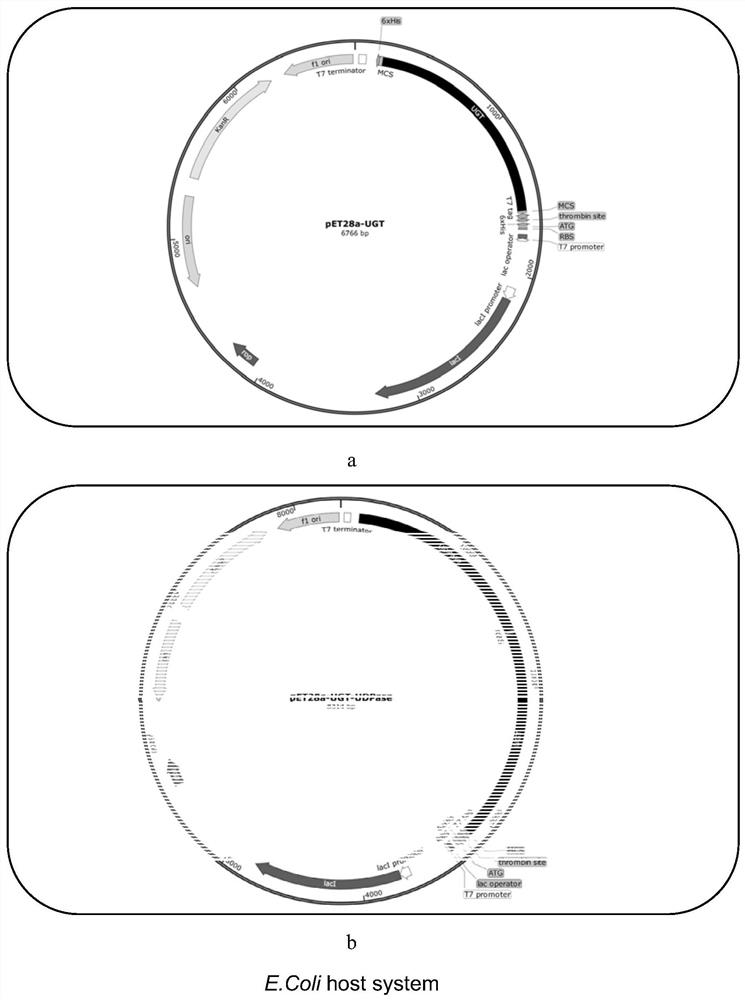
[0024] Construction of embodiment 1pET28a-UGT plasmid and Escherichia coli recombinant strain
[0025] Construction method of pET28a-UGT plasmid:
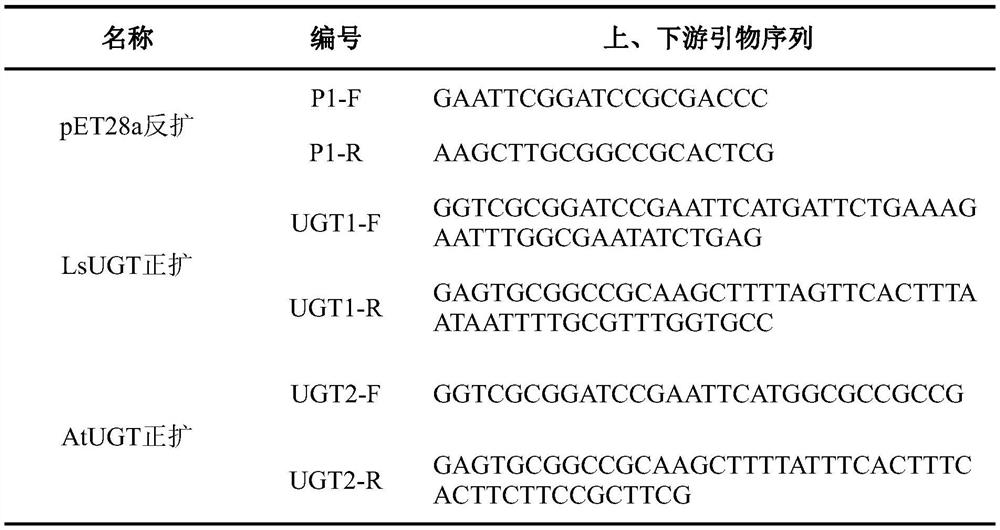
[0026] (1) Using the pET28a vector as a template, PCR amplification was performed using specific primers. The pET28a vector is a commercial vector, purchased from Novagen; the primer sequences are shown in Table 1; after the PCR product is recovered, the linearized vector pET28a-reverse amplification is obtained, and the linearized vector fragment size is 5369bp;
[0027](2) According to the NCBI (https: / / www.ncbi.nlm.nih.gov / ) database published salmon sea lice (Lepeophtheirus salmonis), Arabidopsis thaliana (Arabidopsis thaliana), cotton (Gossypium hirsutum), radish (Raphanus sativus), fruit Fly (Drosophila simulans) UGT protein sequence of 5 kinds of organisms, protein sequence as shown in SEQ ID NO.2, SEQ ID NO.4, SEQ ID NO.6, SEQ ID NO.8 or SEQ ID NO.10, according to Escherichia coli codon preference for codon optimization, ...
Embodiment 2
[0037] Induced expression of embodiment 2 target protein
[0038] Inoculate 5 kinds of engineered bacteria into 5mL LB liquid medium and culture overnight at 37°C and 200rpm. Then, 0.8 mL of the overnight culture was inoculated into 20 mL of LB liquid medium, and cultured at 37° C. and 200 rpm until the OD600 of the bacteria was 0.6. Then IPTG was added to a final concentration of 1 mM, and incubated at room temperature for 16 h to induce the expression of the target protein. After induction, collect the bacteria by centrifugation at 10,000×g for 5 min, and resuspend the bacteria in 1.5 mL of a mixture (50 mM Tris HCl (pH 7.0), 15% (vol / vol) glycerol, 0.1 mM EDTA and 5 mM β-mercaptoethanol) , sonicate for 3 min to disrupt the cells. Subsequently, the Escherichia coli cell lysate was centrifuged at 20,000×g for 10 min, and the supernatant was collected to obtain a crude enzyme solution for enzyme characterization. Crude enzyme solutions were stored at -20°C until further ana...
Embodiment 3
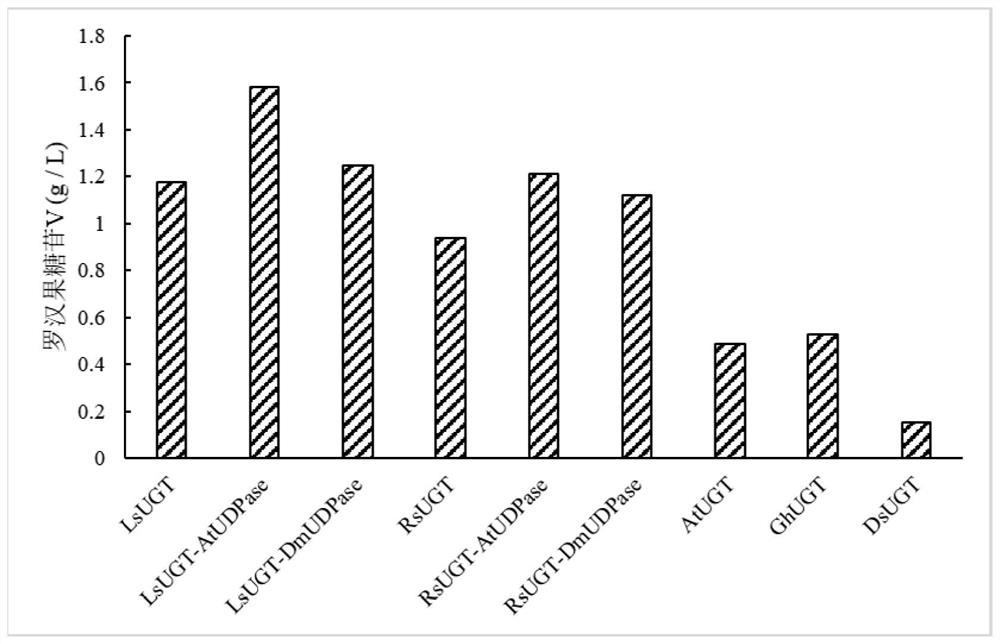
[0039] Example 3 Determination of Mogroside V Content
[0040] Mogrosol was dissolved in 50% DMSO to 20 mM. Add 50 mM Tris HCl (pH 7.0, containing 5 mM β-mercaptoethanol), 25 μL of crude enzyme solution, 8 mM UDP-glucose and 2 mM Mogrosanol to prepare a reaction system with a volume of 100 μL for the determination of UGT enzyme activity. After incubating the reaction solution at 37°C for 24 h, the reaction was terminated by adding 300 μL of methanol solution, and then vortexed briefly. Subsequently, the reaction solution was centrifuged at 20,000×g for 10 minutes, and the supernatant was collected, and the subsequent product content analysis was as follows:
[0041] (1) Add 1 mL of chromatographic grade methanol solution to 1 mL of the above reaction solution to obtain a product lysate;
[0042] (2) After the product lysate was centrifuged at 5000rpm for 10min, the supernatant was collected;
[0043] (3) The supernatant was filtered with a 0.22 μm filter membrane into a bro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com