Preparation method of ethyl 5-chloro-2-fluoro-3-hydroxybenzoate
A technology of ethyl hydroxybenzoate and concentrated sulfuric acid, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., which can solve the problems of long reaction steps, inability to achieve amplification, complex post-processing, and operational uncertainty and other issues, to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
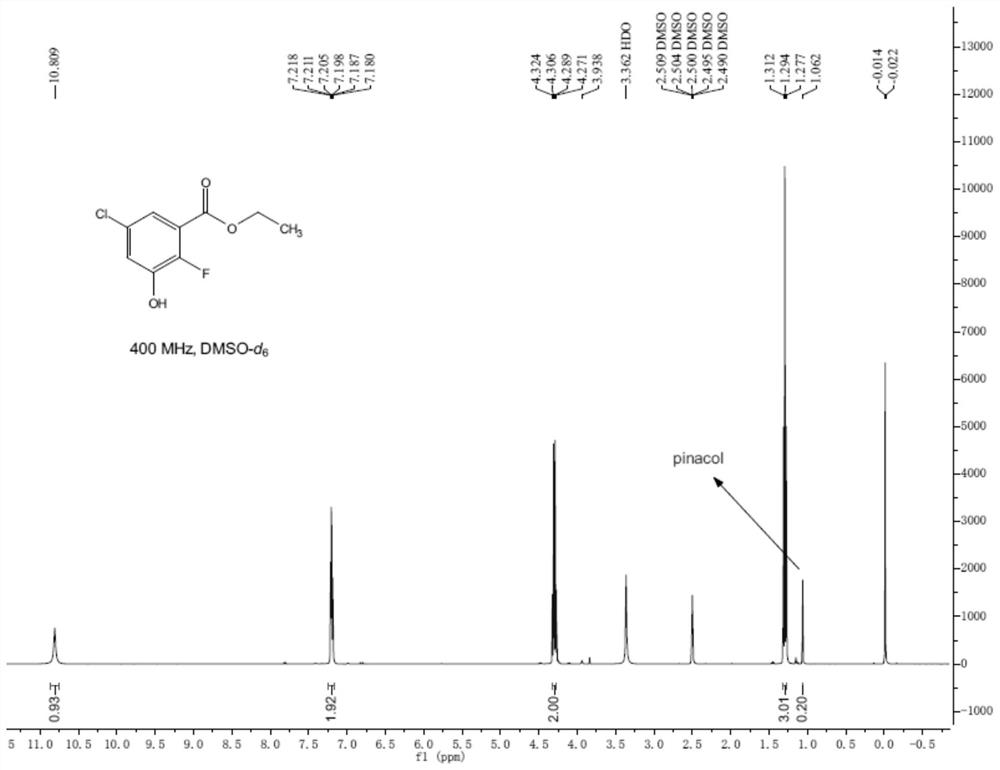
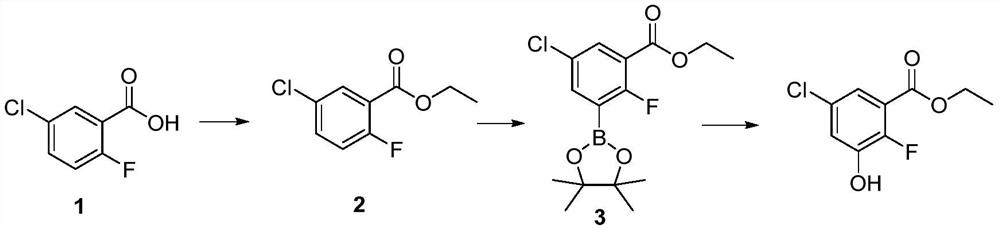
[0025] A kind of preparation method of 5-chloro-2-fluoro-3-hydroxybenzoic acid ethyl ester, described preparation method comprises the steps:
[0026] (1) Dissolve 2-chloro-5-fluorobenzoic acid (65g, 0.37mol) in solvent ethanol (650mL), slowly add concentrated sulfuric acid (65mL) dropwise at room temperature, heat to reflux and stir for 18h, After the reaction is complete, pour the reaction solution into crushed ice and keep stirring, then use 15% NaOH aqueous solution to adjust the pH value to greater than 7, then extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate and concentrate 47 g of ethyl 5-chloro-2-fluorobenzoate was obtained as a yellow oil, with a yield of 63% and a content of 96%.
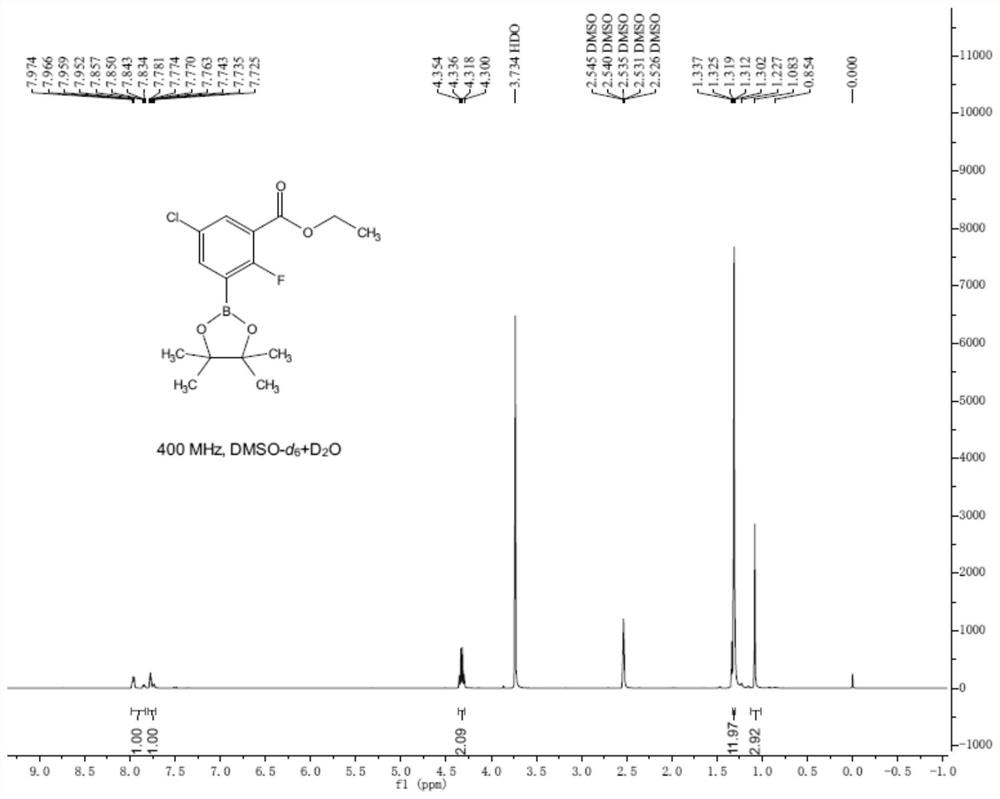
[0027] (2) Ethyl 5-chloro-2-fluorobenzoate (20g, 99mmol), 4,4'-di-tert-butyl-2,2'-dipyridine and (0.8g, 3mmol), methoxy ( Cyclooctadiene) iridium dimer (0.98g, 1.5mmol) and diboronic acid pinacol ester (28g, 108mmol) were added to ...
Embodiment 2
[0032] A kind of preparation method of 5-chloro-2-fluoro-3-hydroxybenzoic acid ethyl ester, described preparation method comprises the steps:
[0033] (1) Dissolve 2-chloro-5-fluorobenzoic acid (65g, 0.37mol) in solvent ethanol (650mL), slowly add concentrated sulfuric acid (325mL) dropwise at room temperature, heat to reflux and stir for 18h, After the reaction is complete, pour the reaction solution into crushed ice and keep stirring, then use 15% NaOH aqueous solution to adjust the pH value to greater than 7, then extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate and concentrate , 50 g of ethyl 5-chloro-2-fluorobenzoate was obtained as a yellow oil, with a yield of 67% and a content of 96%.
[0034] (2) Ethyl 5-chloro-2-fluorobenzoate (20g, 99mmol), 4,4'-di-tert-butyl-2,2'-dipyridine and (5.3g, 20mmol), methoxy ( Cyclooctadiene) iridium dimer (6.5g, 9.9mmol) and diboronic acid pinacol ester (50.9g, 196mmol) were adde...
Embodiment 3
[0037] A kind of preparation method of 5-chloro-2-fluoro-3-hydroxybenzoic acid ethyl ester, described preparation method comprises the steps:
[0038](1) Dissolve 2-chloro-5-fluorobenzoic acid (65g, 0.37mol) in solvent ethanol (650mL), slowly add concentrated sulfuric acid (162mL) dropwise at room temperature, heat to reflux and stir for 18h, After the reaction is complete, pour the reaction solution into crushed ice and keep stirring, then use 15% NaOH aqueous solution to adjust the pH value to greater than 7, then extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate and concentrate 47 g of ethyl 5-chloro-2-fluorobenzoate was obtained as a yellow oil, with a yield of 63% and a content of 96%.
[0039] (2) Ethyl 5-chloro-2-fluorobenzoate (20g, 99mmol), 4,4'-di-tert-butyl-2,2'-bipyridine and (2.7g, 10mmol), methoxy ( Cyclooctadiene) iridium dimer (3.3g, 5mmol) and diboronic acid pinacol ester (25.5g, 98mmol) were added to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com