A kind of preparation method of 1,4 enyne compounds
A compound and enyne technology, applied in the field of organic chemical synthesis, can solve the problems of unfriendly environment and poor economy, and achieve the effects of low preparation cost, convenient operation and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) In a 10 mL Shrek tube, under nitrogen atmosphere, add 0.4 mmol of methyl 2-(hydroxy(phenyl) meth)acrylate, 0.6 mmol of phenylpropioic acid, and 0.015 mmol of tetrakis(triphenylphosphine) palladium , 0.04mmol calcium bis-trifluoromethanesulfonimide, 0.04mmol cesium fluoride, add 4mL dimethyl sulfoxide, stir the reaction under nitrogen and 100 ° C, the reaction equation is:
[0031]
[0032] (2) After the TLC monitoring reaction was complete, the solvent was removed with a vacuum rotary evaporator, and the product was separated by thin-layer chromatography. The developing solvent was petroleum ether / ethyl acetate system, and the product was a yellow liquid (Z)-2-benzylidene- 5-Phenylpentan-4-acid methyl ester, yield 79%.
Embodiment 2
[0034] (1) In a 10mL Shrek tube, under nitrogen atmosphere, add 0.3mmol 1-(4-methylphenyl)prop-2-en-1-ol, 0.45mmol 3-(3-methoxyphenyl) ) propynoic acid, 0.02 mmol tetrakis(triphenylphosphine) palladium, 0.03 mmol calcium trifluoromethanesulfonate, 0.03 mmol cesium fluoride, add 2 mL N,N-dimethylacetamide, stir under nitrogen and 80°C The reaction equation is:
[0035]
[0036] (2) After the TLC monitoring reaction is complete, the solvent is removed with a vacuum rotary evaporator, and the product is separated by thin layer chromatography. The developing solvent is petroleum ether / ethyl acetate system, and the product is a pale yellow liquid (E)-4-methylbenzene yl-5-phenyl-1-penten-4-yne in 67% yield.
Embodiment 3
[0038] (1) In a 10 mL Shrek tube, under a nitrogen atmosphere, add 0.35 mmol of cinnamyl alcohol, 0.5 mmol of 3-(3,4-dimethoxyphenyl) propynoic acid, and 0.015 mmol of tetrakis(triphenylphosphine) Palladium, 0.035mmol calcium bis-trifluoromethanesulfonimide, 0.035mmol cesium fluoride, add 3mL ethylene glycol dimethyl ether, and stir the reaction under nitrogen and 90°C. The reaction equation is:
[0039]
[0040] (2) After the TLC monitoring reaction is complete, the solvent is removed with a vacuum rotary evaporator, the product is separated by thin layer chromatography, the developing solvent is petroleum ether / ethyl acetate system, and the product is a pale yellow liquid (E) 1,5-diphenyl yl-1-penten-4-yne, 76% yield.
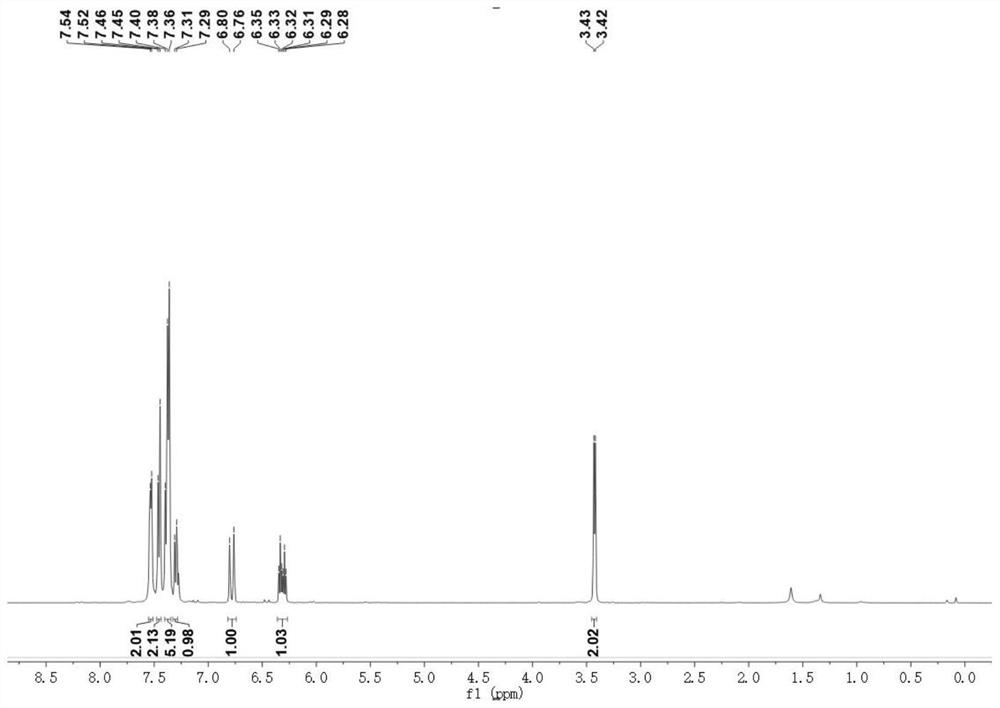
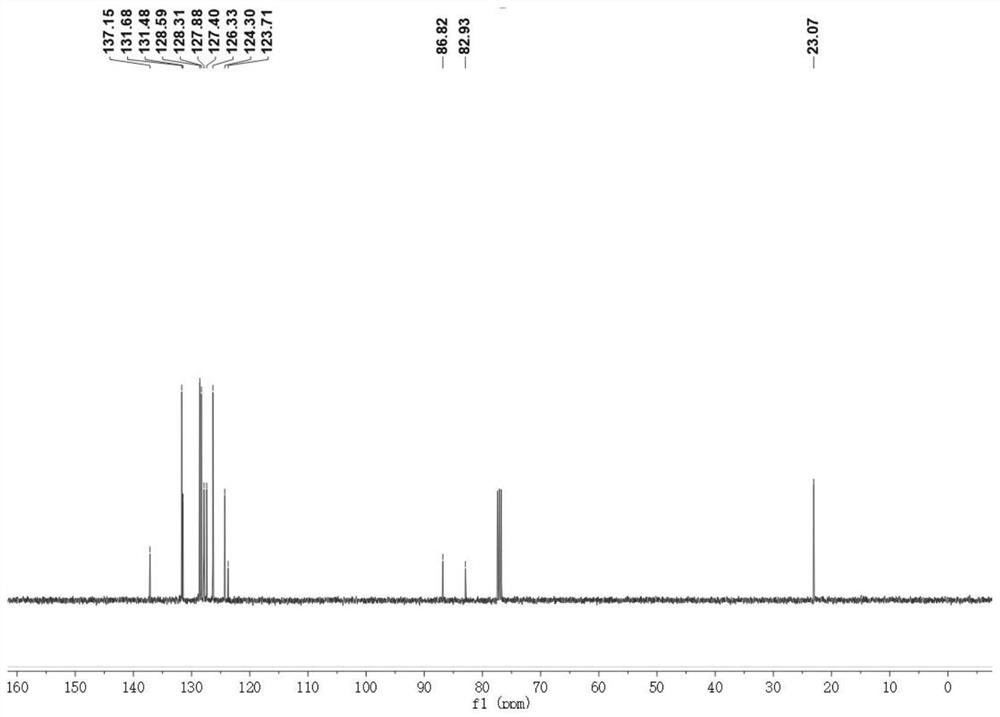
[0041] (E) 1,5-diphenyl-1-penten-4-yne was detected by nuclear magnetic resonance, as shown in the appendix Figures 1 to 2 shown, attached figure 1 is the H NMR spectrum of (E) 1,5-diphenyl-1-penten-4-yne, with figure 2 is the carbon nuclear magnetic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com