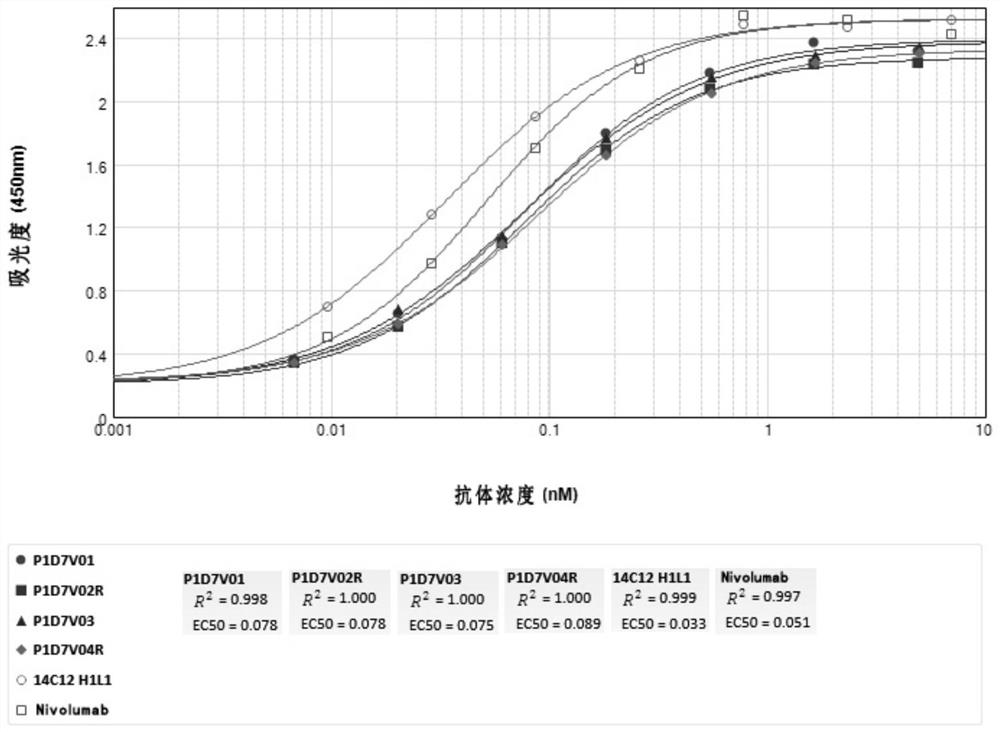
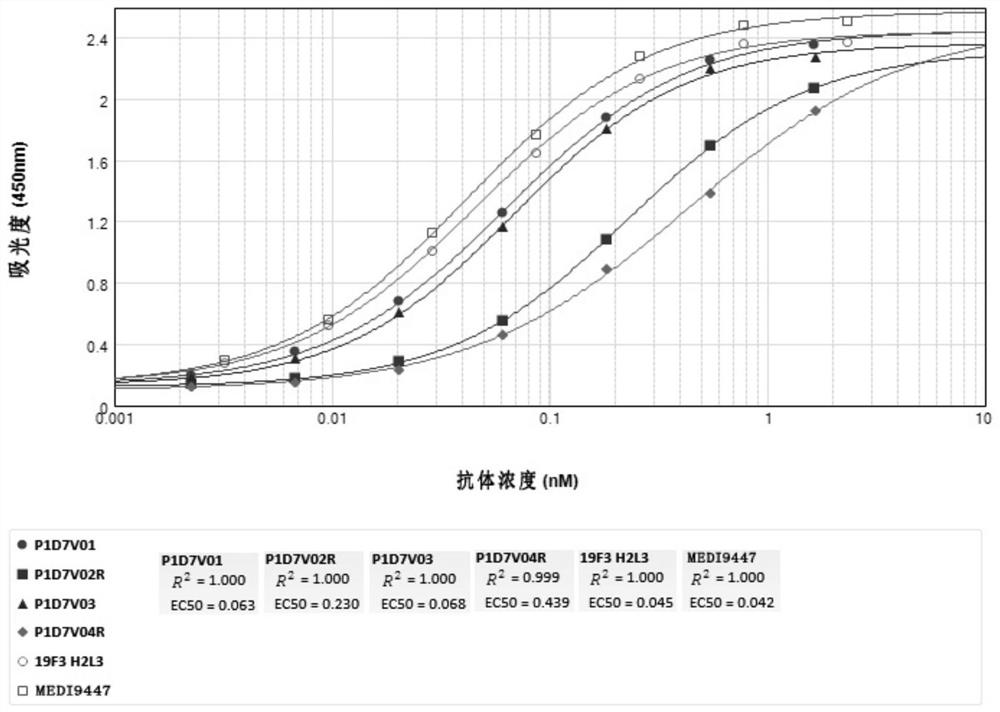
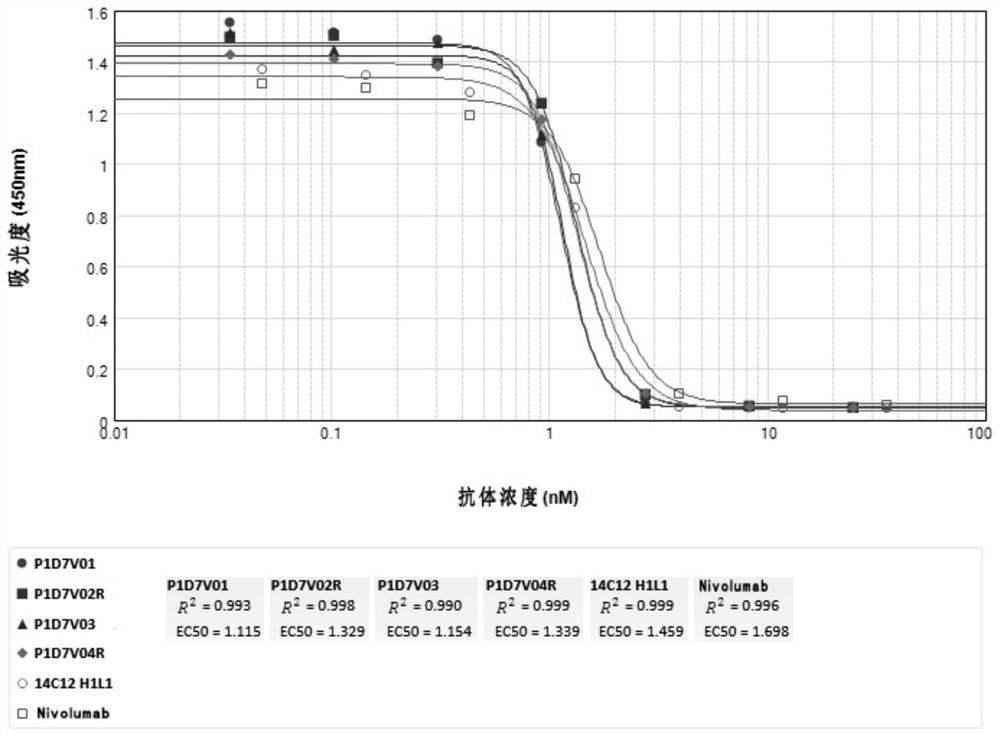
Anti-CD73-anti-PD-1 bispecific antibody and application thereof
A bispecific antibody, CD73- technology, applied in the field of tumor therapy and molecular immunology, to increase the safety and efficacy, reduce the production of adenosine, and relieve the effects of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] Example 1: Preparation of anti-CD73 antibody 19F3
[0263] 1. Preparation of hybridoma cell line LT014
[0264] The antigen used to prepare the anti-CD73 antibody is human NT5E-his (NT5E is Genbank ID: NP_002517.1, position: 1-552). The splenocytes of the immunized mice were fused with mouse myeloma cells to make hybridoma cells, and human NT5E (NT5E is GenbankID: NP_002517.1, position: 1-552)-Biotin was used as an antigen to conduct hybridoma cells Screening by indirect ELISA method to obtain hybridoma cells that can secrete antibodies specifically binding to CD73. For the hybridoma cells screened by the ELISA method, a stable hybridoma cell line was obtained through the limiting dilution method. The above hybridoma cell line was named hybridoma cell line LT014, and the monoclonal antibody secreted by it was named 19F3.
[0265] Hybridoma cell line LT014 (also known as CD73-19F3), which was deposited in the China Center for Type Culture Collection (CCTCC) on June 21...
Embodiment 2
[0268] Example 2: Sequence Analysis of Anti-CD73 Antibody 19F3
[0269] mRNA was extracted from the LT014 cell line cultured in Example 1 according to the method of the Bacteria Total RNA Extraction Kit (Tiangen, Cat. No. DP430).
[0270] According to Invitrogen III First-Strand Synthesis System for RT-PCR Kit Instructions Synthesize cDNA and perform PCR amplification.
[0271] The PCR amplification product was directly cloned by TA, and the specific operation was performed referring to the instructions of the pEASY-T1 Cloning Kit (TransgenCT101) kit.
[0272] The products of TA clones were directly sequenced, and the sequencing results were as follows:
[0273] The nucleic acid sequence (363bp) of the heavy chain variable region of 19F3 is shown in SEQ ID NO:1, and the encoded amino acid sequence (121aa) is shown in SEQ ID NO:2.
[0274] According to the IMGT numbering system, the sequence of the heavy chain CDR1 is shown in SEQ ID NO:3, the sequence of the heavy chain CD...
Embodiment 3
[0278] Example 3: Design, preparation and detection of humanized antibodies against human CD73
[0279] 1. Design of light chain and heavy chain sequences of humanized antibodies 19F3H2L3 and 19F3H2L2
[0280]According to the three-dimensional crystal structure of human CD73 protein (Hage T, Reinemer P, Sebald W. Crystals of a1:1 complex between human interleukin-4 and the extracellular domain of its receptor alpha chain. Eur J Biochem.1998; 258 (2): 831-6 ) and the sequence of the antibody murine antibody 19F3 obtained in Example 2, the antibody model was simulated by computer, and mutations were designed according to the model to obtain the variable region sequences of antibodies 19F3H1L1, 19F3H2L3 and 19F3H2L3, and the corresponding heavy chain variable region sequences were respectively 19F3H1, 19F3H2 (the amino acid sequences are shown in SEQ ID NO: 93, SEQ ID NO: 97), the light chain variable region sequences are respectively 19F3L1, 19F3L2, 19F3L3 (the amino acid sequen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com