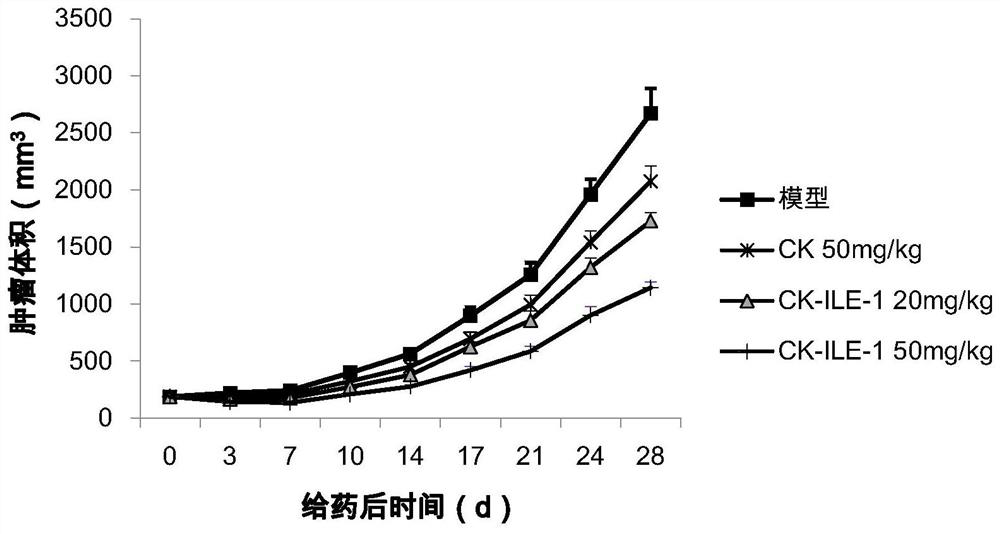
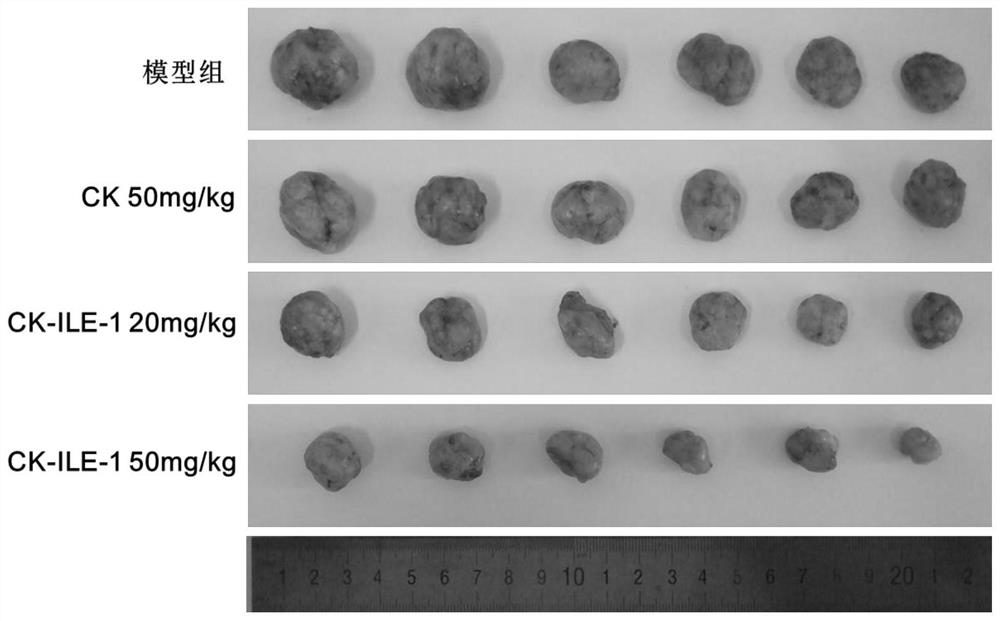
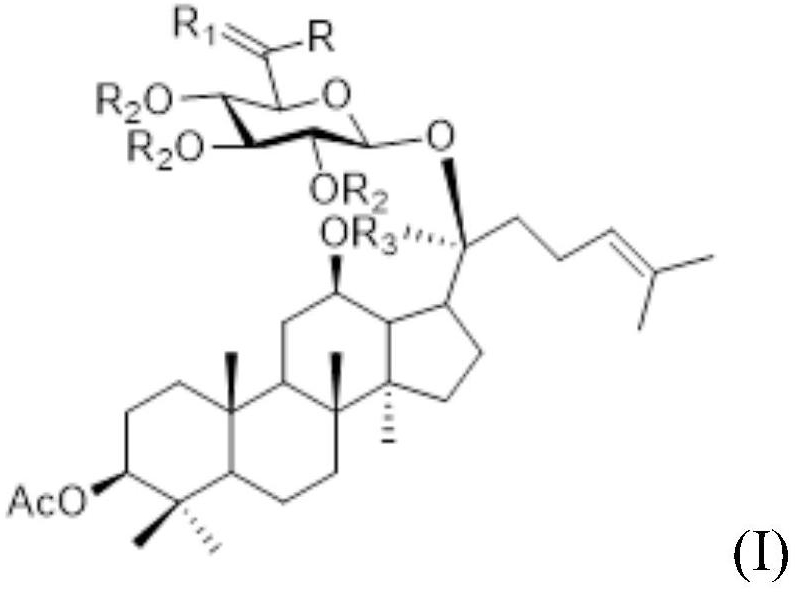
Ginsenoside CK derivative and application thereof in preparation of antitumor drugs
A technology of ginsenosides and derivatives, applied in the direction of antineoplastic drugs, drug combinations, glycoside steroids, etc., can solve the problems of limited CK sources, poor water solubility of ginsenoside CK derivatives, difficult separation and purification, etc., and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1 synthetic compound CK-1
[0096] Weigh 500mg of ginsenoside CK with an electronic balance, dissolve it in 25mL of anhydrous dichloromethane, add 5mL of triethylamine and 1mL of tert-butyldiphenylchlorosilane in turn at 0°C, keep this temperature for 10min, and naturally rise to Reaction at room temperature for 8h. After thin-layer chromatography TLC (D / M=10:1) monitoring is complete, add saturated ammonium chloride to stop the reaction, extract 3 times with 25mL dichloromethane, the obtained organic phase is washed with saturated brine, dried over anhydrous sodium sulfate, filtered, Concentrated under reduced pressure, the obtained concentrate was purified by silica gel column chromatography (D / M=10:1), and the obtained product CK-1 (600 mg, yield 88.4%) was a white solid. MS(ESI): calcd for C 52 h 81 o 8 Si[M+H] + 861.5695, found 861.5678.
Embodiment 2
[0097] Embodiment 2 synthetic compound CK-2 and CK-2-2
[0098] Weigh 136mg of ginsenoside CK-1 with an electronic balance, then weigh 10mg of 4-dimethylaminopyridine (DMAP), dissolve it in 3mL of triethylamine and 5mL of dichloromethane, and fully stir , into the nitrogen protection. Then place the three-necked round-bottomed flask under ice bath conditions, and add 0.30 mL of acetic anhydride AC to the reaction solution. 2 O, after 10 min, it was raised to room temperature to continue the reaction, and the reaction progress was monitored by TLC (P / E=1:1). After reacting for 6 hours, add saturated ammonium chloride to stop the reaction, extract 3 times with 5 mL of dichloromethane, wash the obtained organic phase with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure, and the obtained concentrate is subjected to silica gel column chromatography After purification (P / E=3:1), the obtained products CK-2 (131 mg, yield 77.5%) and...
Embodiment 3
[0101] Embodiment 3 synthetic compound CK-2A
[0102] Take by weighing the ginsenoside CK of 100mg with electronic balance, then weigh the 4-dimethylaminopyridine (DMAP) of 12mg, it is dissolved in the dichloromethane of the triethylamine of 2.5mL and 5mL, and carry out sufficient stirring, Introduce nitrogen protection. Then place the three-necked round-bottomed flask under ice bath conditions, and add 0.30 mL of acetic anhydride AC to the reaction solution. 2 O, rose to room temperature after 10 min to continue the reaction. Thin-layer chromatography TLC (P / E=1:1) monitors that the reaction is complete, add saturated ammonium chloride to stop the reaction, extract 3 times with 5mL dichloromethane, wash the obtained organic phase with saturated brine, dry over anhydrous sodium sulfate, and filter , concentrated, and the obtained concentrate was purified by silica gel column chromatography (P / E=2:1), and the obtained product CK-2A (130 mg, yield 92.8%) was a white solid. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com