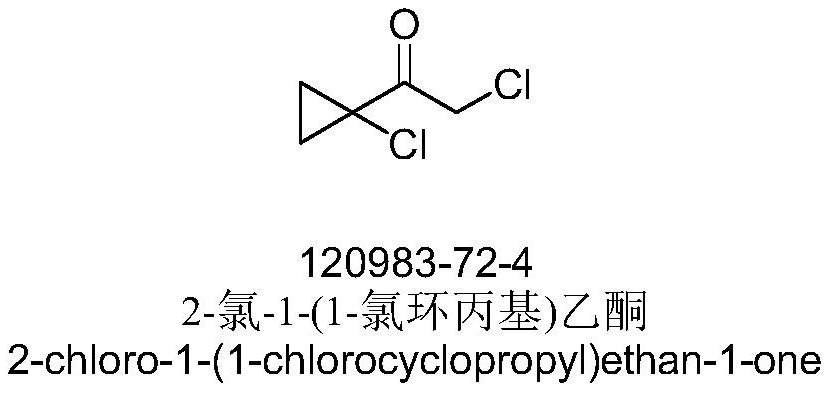
Synthesis method of high-content 2, 2-dichloro-1-(1-chlorocyclopropyl) ethanone for quantitative and qualitative analysis
The technology of chlorocyclopropyl ethyl ketone and cyclopropyl ethyl ketone is applied in the field of synthesis of high-content 2,2-dichloro-1-ethyl ketone, so as to reduce the impact of environmental pollution and human health, and achieve high purity. , the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone:
[0026]
[0027] Add 1-(1-chlorocyclopropyl)ethanone (59.3g, 0.50mol), methanol (MeOH) 30mL and dichloromethane (DCM) 180mL in a reaction flask equipped with a thermometer, magnetic stirring and acid tail gas absorption device , Chlorine gas (177.3g, 2.50mol) was introduced under stirring at 10-20°C, and the introduction was completed after 18 hours, and then the reaction was continued for 6 hours to end the reaction. Slowly add purified water to the reaction system under cooling and stirring in an ice bath, separate the liquids, wash the organic phase with saturated sodium bicarbonate solution and water respectively, dry with molecular sieves, and evaporate the solvent dichloromethane and methanol after heating and atmospheric pressure. 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone was distilled under pressure conditions of 120°C, 50mmHg).
[0028] The obtained product is a colorless liquid, weight: 74.5 g;...
Embodiment 2
[0030] Synthesis of 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone
[0031]
[0032] Add 1-(1-chlorocyclopropyl)ethanone (118.6g, 1.0mol), methanol (60mL) and dichloromethane (360mL) in a reaction flask equipped with a thermometer, mechanical stirring and acid tail gas absorption device, Sulfonyl chloride (539.8 g, 4.0 mol) was added dropwise under stirring at 0-10°C, and the addition was completed after 12 hours, and the temperature was continued for 12 hours to terminate the reaction. Slowly add purified water to the reaction system under cooling and stirring in an ice bath, separate the liquids, wash the organic phase with saturated sodium bicarbonate solution and water respectively, dry over molecular sieves, and distill under reduced pressure (collect 118-123 ° C fractions under 45-50 mmHg pressure ) to give 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone.
[0033] The weight of the obtained product: 152.3g; the purity: 97.8%; the yield: 81.3%.
Embodiment 3
[0035] Synthesis of 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone
[0036]
[0037]Add 1-(1-chlorocyclopropyl)ethanone (59.3g, 0.50mol) and dichloroethane (1200mL) into a reaction flask equipped with a thermometer, magnetic stirring and acid tail gas absorption device, Phosgene (296.7 g, 3.00 mol) was introduced under stirring, and the introduction was completed after 18 hours, and the temperature was continued for 12 hours to end the reaction. Slowly add purified water to the reaction system under cooling and stirring in an ice bath, separate the liquids, wash the organic phase with saturated sodium bicarbonate solution and water respectively, dry over molecular sieves, and distill under reduced pressure (collect 118-123 ° C fractions under 45-50 mmHg pressure ) to give 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone.
[0038] The weight of the obtained product: 73.2g; the purity: 97.4%; the yield: 78.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com