Antibacterial cationic injectable hydrogel dressing and preparation method thereof
A technology for injecting water and cations, which can be used in pharmaceutical formulations, bandages, and drug delivery. It can solve the problems of unstable Schiff base bonds and poor mechanical strength, and achieve improved mechanical strength, good biocompatibility, and good promotion. The effect of repair ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The invention provides a kind of preparation method of antibacterial cationic injectable hydrogel dressing, comprises the following steps:
[0045] a) The multi-arm polyethylene glycol terminated by phthalaldehyde is dissolved in a solvent to obtain solution A;
[0046] b) the cationic polymer is dissolved in a solvent to obtain solution B;
[0047] c) mixing and reacting the solution A and the solution B to obtain an antibacterial cationic hydrogel dressing;
[0048] The steps a) and b) are not limited in sequence.
[0049] Regarding step a): The ortho-phthalaldehyde-terminated multi-armed polyethylene glycol is dissolved in a solvent to obtain solution A.
[0050] In the present invention, the multi-arm polyethylene glycol terminated by phthalaldehyde is selected from one or more of the compounds shown in formula 1 to formula 4:
[0051]
[0052] in:
[0053] p, n, x, m are degrees of polymerization;
[0054]35≤p≤133, 25≤n≤100, 17≤x≤75, 12≤m≤50. If the above-...
Embodiment 1
[0076] 1. Sample preparation:
[0077] S1. At 37°C, dissolve the four-armed polyethylene glycol (n=63) capped with phthalaldehyde shown in formula 2 in PBS buffer solution with pH=7.4 to prepare a solution with a mass fraction of 5%; then , the pH of the system was adjusted to 7.4 with NaOH solution to obtain solution A.
[0078] S2. Dissolve ε-poly L-lysine (molecular weight 5000) in PBS buffer solution with pH=7.4 at 37°C to prepare a solution with a mass fraction of 5%; then, adjust the pH of the system to 7.4, to obtain solution B.
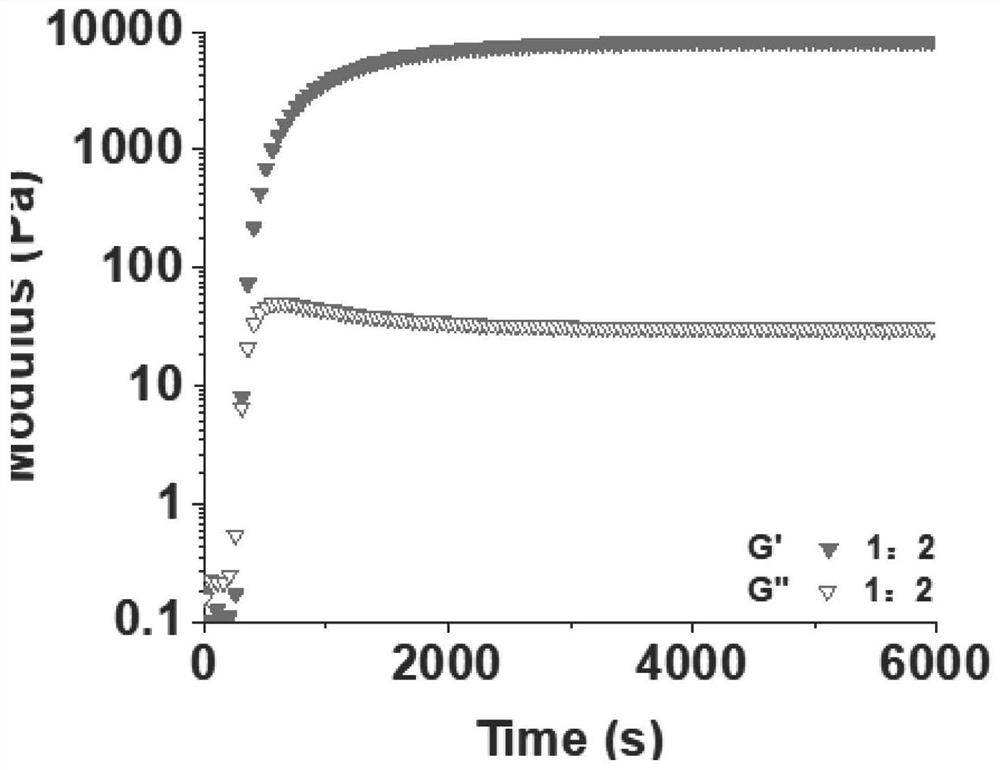
[0079] S3. The mass ratio of multi-armed polyethylene glycol capped with cationic polymer: o-phthalaldehyde = 1: 2, mix solution A and solution B at 37°C, and vortex for 5s to obtain antibacterial cationic hydrogel Gum dressing.
[0080] 2. Sample test:
[0081] (1) Gel forming time:
[0082] The test tube inversion method was used to measure the gelation time of the sample at 37°C, and the results showed that the gelation time of the sam...
Embodiment 2
[0091] 1. Sample preparation:
[0092] Carry out according to the preparation process of embodiment 1, difference is, in step S2, the molecular weight of ε-poly L-lysine is 18000.
[0093] 2. Sample test:
[0094] The test was carried out according to the test method of Example 1, and the result showed that the gelation time was 175±7s. The modulus of elasticity is 9500Pa, see Figure 5 , Figure 5 It is the effect figure of the mechanical properties of the antibacterial cationic hydrogel dressing obtained in embodiment 2. The degradation time is 15d, see Figure 6 , Figure 6 It is the in vitro degradability effect figure of embodiment 2 gained antibacterial cationic hydrogel dressing. Antibacterial live-death test results show: Staphylococcus aureus all died, the results see Figure 7 , Figure 7It is the antibacterial effect figure of embodiment 2 gained antibacterial cationic hydrogel dressing to Staphylococcus aureus.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mechanical strength | aaaaa | aaaaa |
| mechanical strength | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com