Preparation method of trifluoroacetophenone derivative
A technology for trifluoroacetophenone and derivatives, which is applied in the field of preparation of trifluoroacetophenone derivatives, can solve the problems of high energy consumption, unsuitability for industrial production, etc. produce less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
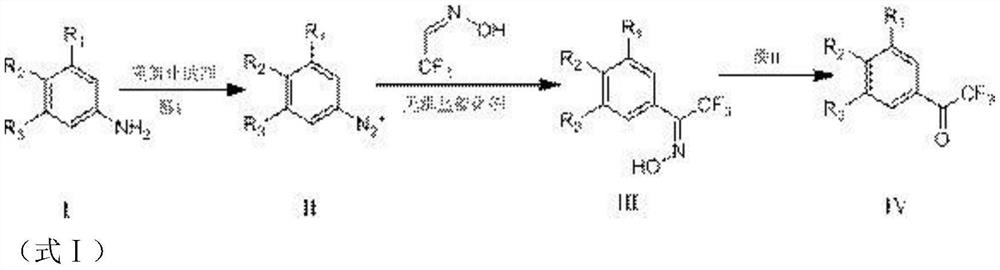
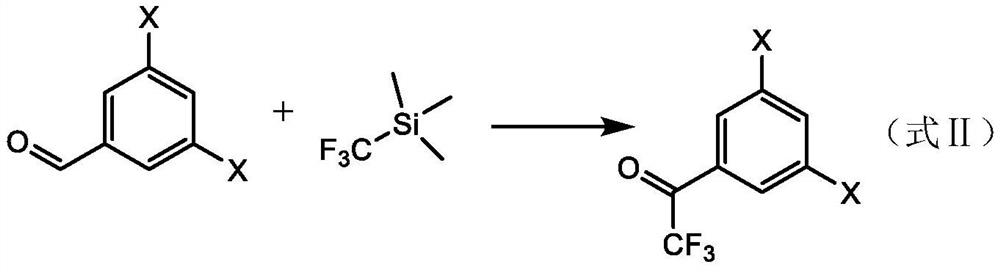
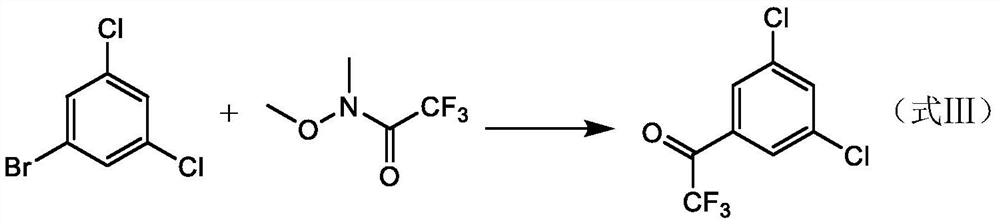
[0048] Example 1, a method of preparing a trifluorophenylidene derivative (3,5-dichloro-trifluorophenylidezone), and its reaction formula is shown in formula I-A.
[0049]
[0050] The specific reaction of the reaction includes the steps of:
[0051] S1, weighing compound I0.3 mol (48.6 g), slowly dripping in a 300 g of sulfuric acid solution (containing acid I (sulfuric acid) in 0.61 mol), and then held stirring during the dropwise addition. After completion, stirring was stirred for 20 min, then cooled to -5 ± 5 ° C, and 76 g of a 20% aqueous solution (containing diazotization reagent (sodium nitrite) was added dropwise, and the dropwate was 2 h. After the dropwise addition, the temperature was less than 0 ° C and reacted. After the point plate was monitored until the reactive material point completely disappeared, the reactive nitrogen was obtained, and the atrosity was obtained at 0 ° C.
[0052]S2, 7.5 g of pentaqueous sulfate (inorganic salting catalyst, 0.03 mol), 9.0 g o...
Embodiment 2~17
[0054] Examples 2 to 17, the difference from Embodiment 1 is that the compound I is replaced, and thus, the obtained compound IV has corresponding changes. The amount of the substance to which the compound I was added to the reaction was maintained unchanged, and the other parameters in the reaction were also maintained unchanged, and the yield of the particular compound I and the prepared target product (compound IV) was shown in Table 1.
Embodiment 1~17
[0055] Table 1, Example 1 to 17 Compound yield
[0056]
[0057]
[0058]
[0059] In the above embodiment, the nuclear magnetic resonance spectrum of compound IV in which a part of the compound IV is shown in Table 2.
[0060] Table 2, the structural characterization of partial compounds
[0061]
[0062]
[0063] In the above embodiment, the nuclear magnetic resonance spectrum was determined in deuterated chloroform.
[0064] By the above experimental data, the preparation method in the present application can be used to first achieve a higher reaction yield, and the yield of all reactions is over 65%, and the entire reaction is actually a pot to cook, out In the step S2, there is a step of resetting the liquid, and the remaining time can be carried out in the same reactor, and the subsequent separation is only simply washed, and the solvent is removed. The process required for the reaction process is short, the consumption is less, the condition is mild It is easy to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com