Composition for allergy prevention, atopic dermatitis alleviation or skin regeneration, containing, as active ingredient, undecane or undecanal
A technology of atopic dermatitis and active ingredients, applied in the direction of allergic diseases, active ingredients of aldehydes, skin care preparations, etc., can solve problems such as hand tremors, hair loss, deterioration, and safety of side effects have not been ensured, and reduce inflammation The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
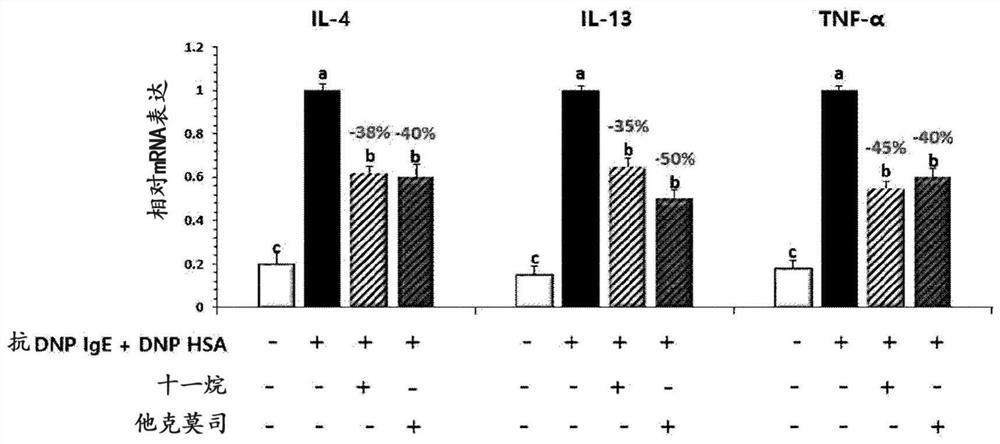
[0100] Example 1: Evaluation of secretion and expression of inflammation-related cytokines after decanogenesis of hypertrophyletal cells and keratinum
[0101] 1-1. Experimental method
[0102] 1) Mermatology
[0103] Mermatic cells (rat basinosycellular leukemia cell line, RBL-2H3) were purchased from ATCC (National Virginia Manasas) and used. DMEM (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) , US) culture cells in the medium. By at 37 ° C, 5% CO 2 Culture was cultured under conditions to test cells. The fat cells were suspended in DMEM containing 10% FBS, then assigned to 1 × 10 in 6-well plates (Corning, US). 6 Cell / ml number of cells.
[0104] Then, it was sensitized with anti-DNP (dinitrobenzene) -iGe (30 ng / ml), and in the incubator at 37 ° C, 5% CO 2 The incubation was cultured for 24 hours. After washing with PBS (phosphate buffered saline), 4 hours were treated with DNP-HSA (dinitro...
Embodiment 2
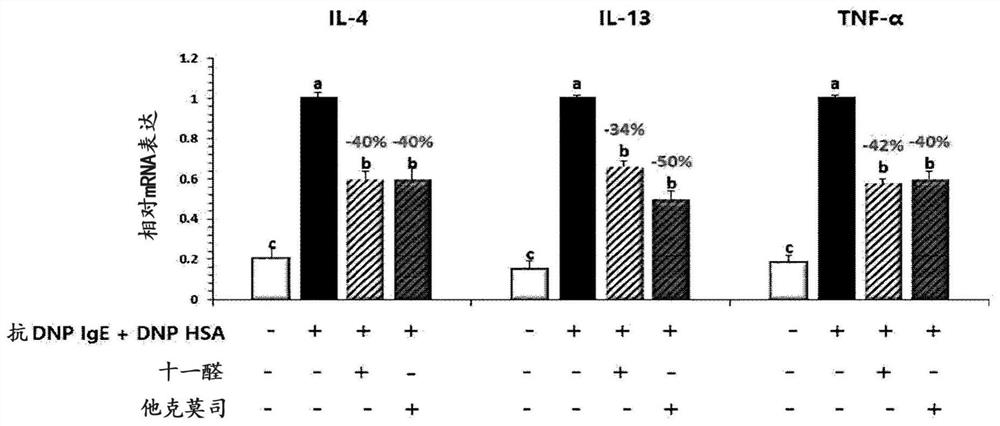
[0116] Example 2: Evaluation of the secretion and expression of inflammation related cytokines after umethral aldehyde infendal cells and keratin forming cells
[0117] 2-1. Experimental Method
[0118] 1) Mermatology
[0119] Mermatic cells (rat basinosycellular leukemia cell line, RBL-2H3) were purchased from ATCC (National Virginia Manasas) and used. DMEM (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) (Gibco BRL) , US) culture cells in the medium. By at 37 ° C, 5% CO 2 Culture was cultured under conditions to test cells. Mermatocytes were suspended in DMEM containing 10% FBS and then assigned to 1 × 106 cells / mL cells in 6-well plates (Corning, US).
[0120] Then, it was sensitized with anti-DNP (dinitrobenzene) -iGe (30 ng / ml), and in the incubator at 37 ° C, 5% CO 2 The incubation was cultured for 24 hours. After washing with PBS (phosphate buffered saline), 4 hours were treated with DNP-HSA (dinitropyr-serum albumin; 10 μg...
preparation Embodiment 1
[0131] [Preparation Example 1] Preparation of the pharmaceutical composition
[0132] Preparation of Powder
[0133] 20mg of decanoalk or eleven aldehyde
[0135] 10mg talc
[0136] The above components were mixed and mounted in an invasive cloth to prepare a powder.
[0137] Preparation of Tablets
[0138] 10mg of uldell or eleven aldehyde
[0139] 100mg corn starch
[0142] After mixing the above ingredient, a tablet is prepared by a method according to a conventional prepared tablet.
[0143] Preparation of Capsules
[0144] 10mg of uldell or eleven aldehyde
[0145] 3MG microcrystalline cellulose
[0147] 0.2mg magnesium stearate
[0148] After mixing the above ingredient, the capsule is prepared in the gelatin capsule according to the conventional preparation capsule.
[0149] Preparation of Injection
[0150] 10mg of uldell or eleven aldehyde
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com