Method for detecting 2, 4-dichlorophenoxyacetic acid
A technology of dichlorophenoxyacetic acid and phosphatase, which is applied in the field of pesticide detection, can solve the problems of time-consuming, long-term consumption, survival rate, reproductive ability decline, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of cerium-pyrophosphate coordination polymer fluorescent nanoparticles (Ce-ppi for short):
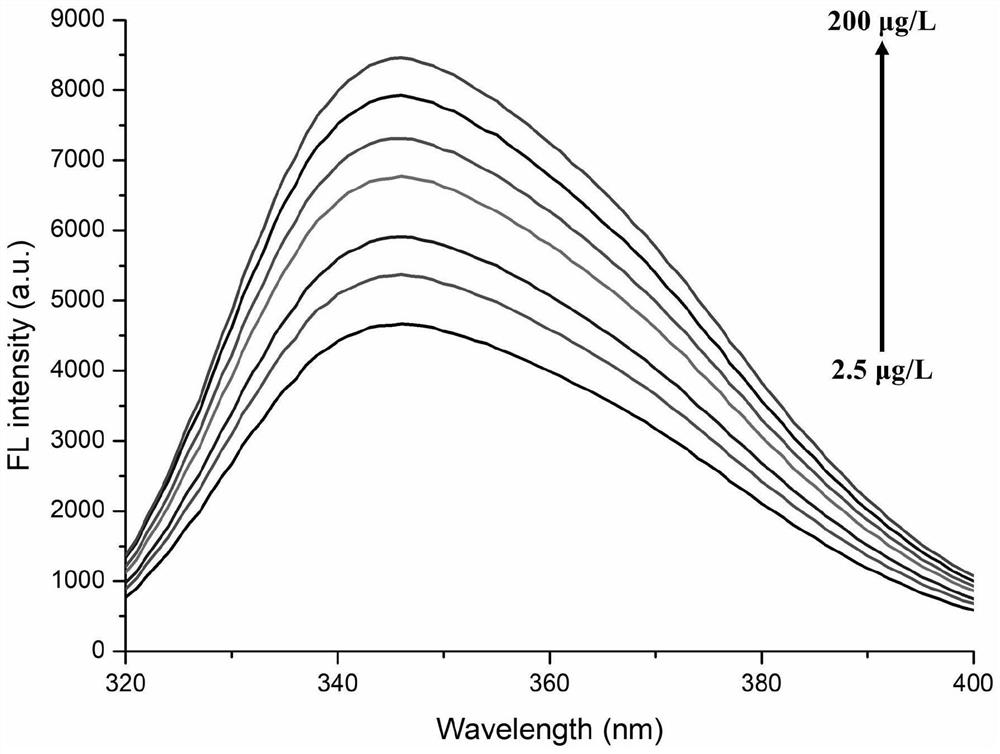
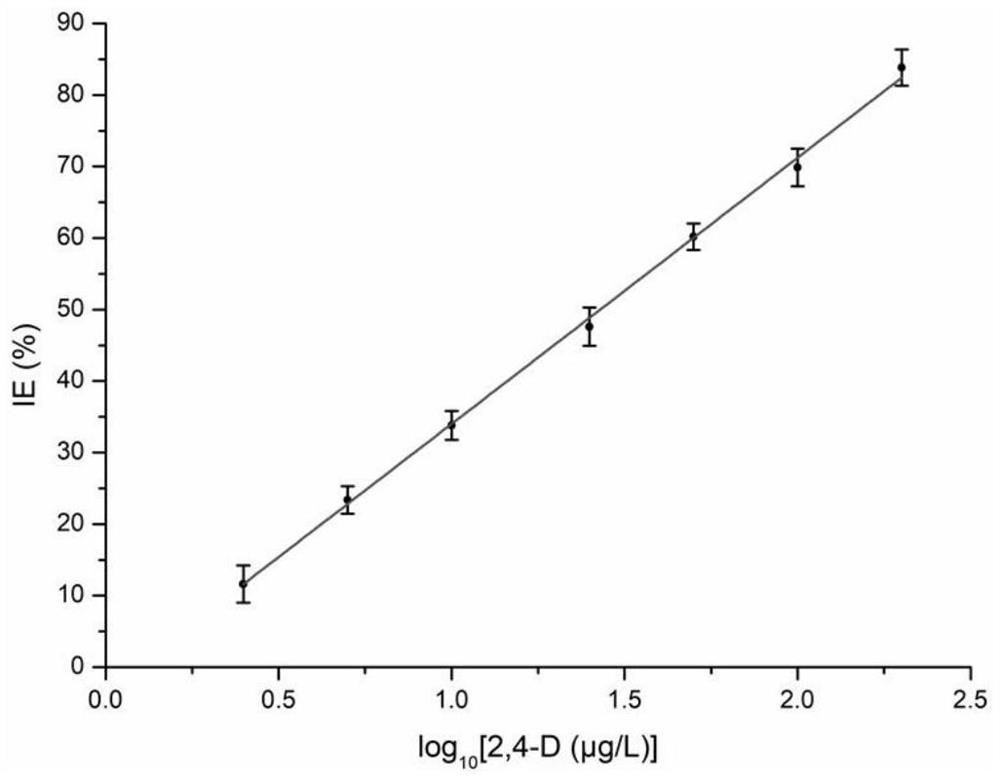
[0053] First, an alkaline phosphatase (ALP for short) solution with a concentration of 100 U / mL was prepared using Tris-HCl buffer (concentration: 50 mM, pH=8.0) as a solvent. 2,4-dichlorophenoxyacetic acid ( Abbreviated as 2,4-D) solution, used to establish a standard curve method for quantitative detection of 2,4-D.
[0054] Take 5 μL of LALP solution in a 2 mL plastic centrifuge tube, then add 25 μL of 2,4-D solution into the centrifuge tube, mix the two thoroughly, and let stand at 37°C for 15 minutes. Afterwards, 10 μL of 1 mM sodium pyrophosphate solution and 20 μL of 50 mM Tris-HCl buffer solution (pH=8.0) were added to the centrifuge tube, mixed thoroughly, and left to react at 37° C. for 25 minutes. After the reaction was completed, 20 μL of 0.5 mM cerium nitrate solution was added to the centrifuge tube, and the total volume of the solution was diluted to 5...
Embodiment 2
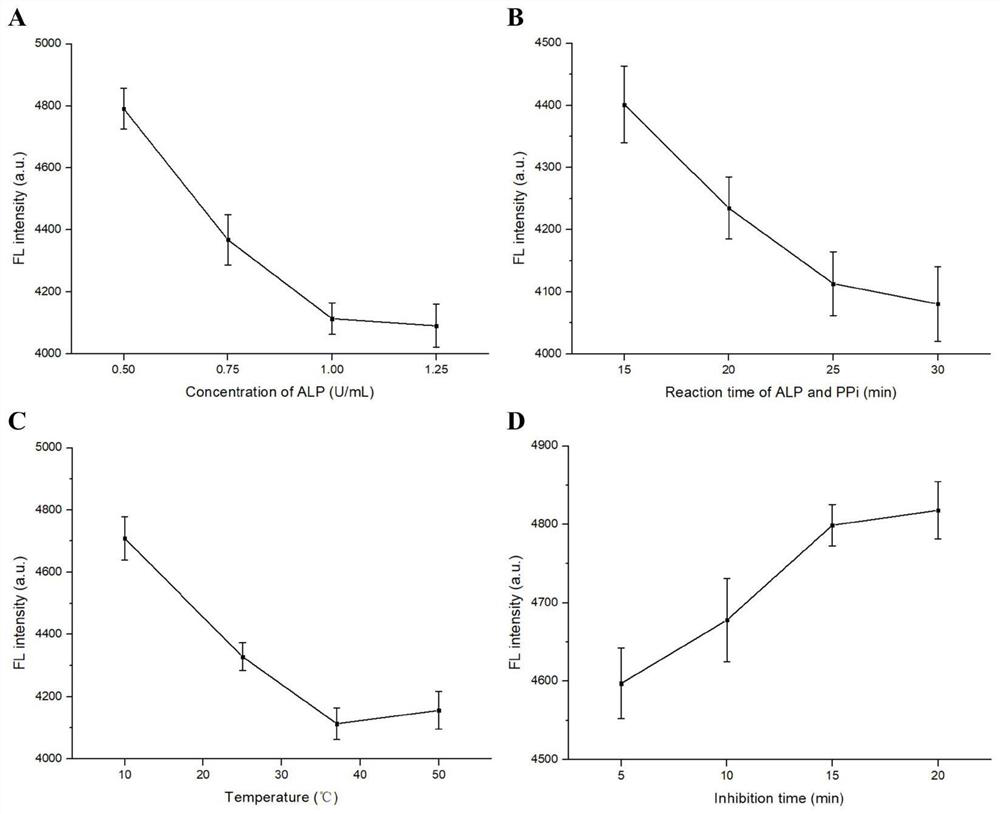
[0057] Adopt the method identical with embodiment 1 to investigate the influence of ALP concentration, standing time, temperature of reaction and reaction time on detection result, result is as follows:
[0058] When the ALP concentration reaches 1.25U / mL, increase the ALP concentration again, the fluorescence intensity of Ce-PPi will not change, indicating that the PPi reaction is complete at this time ( image 3 in A). Therefore, 1U / mL was chosen as the ALP concentration.
[0059] After standing time 25min, the fluorescence intensity of Ce-PPi no longer changes ( image 3 in B). Therefore, 25min was chosen as the time for the mixed reaction of ALP and PPi.
[0060] When the reaction temperature was 37 °C, the fluorescence intensity of Ce-PPi reached the lowest value ( image 3 in C). Therefore, 37°C was selected as the reaction temperature.
[0061] from image 3 In D, it can be seen that when the mixing reaction time of 2,4-D and ALP is 15 min, the fluorescence inten...
Embodiment 3
[0063] Test sample test method:
[0064] The ability of the method to detect 2,4-D residues in real samples (tap water, rice and Chinese cabbage) was validated by the addition recovery method. The tap water was taken from the College of Science, China Agricultural University. After passing through a 0.22 μm filter membrane, the 2,4-D standard solution was diluted to prepare water samples containing different concentrations of 2,4-D (0.05, 0.2 or 0.1 mg / L). During detection, 50 μL of water sample containing 2,4-D was taken, and the remaining detection steps were the same as those for detecting 2,4-D in the standard solution (Example 1). Rice and Chinese cabbage samples were purchased from local supermarkets. The extraction method of 2,4-D residues added in the samples refers to the above-mentioned literature reports. The specific steps are as follows: Accurately weigh 1 g of the mashed samples into a 10 mL plastic centrifuge tube, add 10 μL of 2 , 4-D solution (concentrations ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com