Nitrogen-containing heterocyclic compound as well as preparation method, pharmaceutical composition and application thereof
A technology of nitrogen heterocyclic compounds and compounds, which is applied in the field of nitrogen-containing heterocyclic compounds and their preparation, and can solve the problems of drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
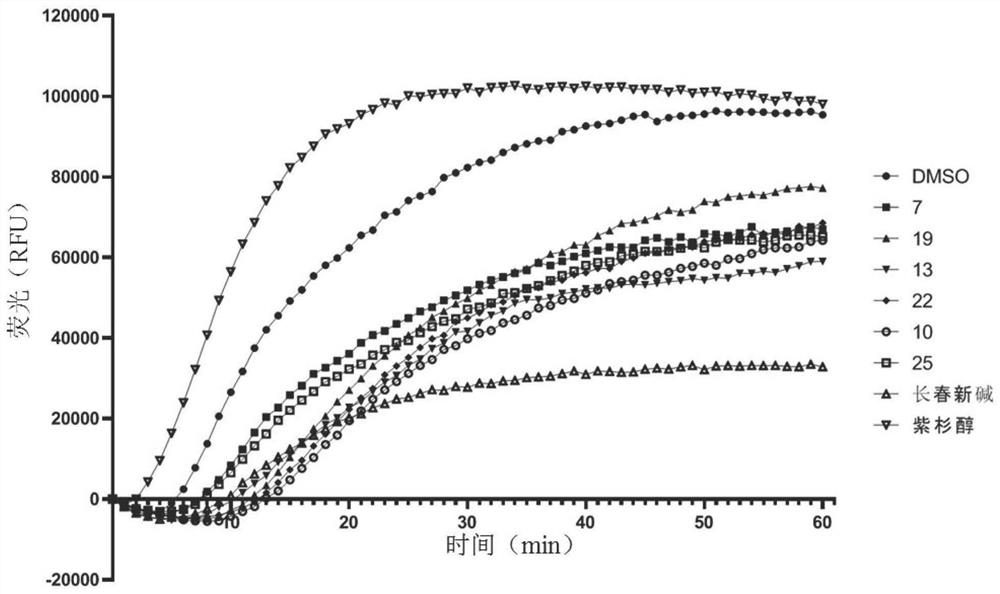
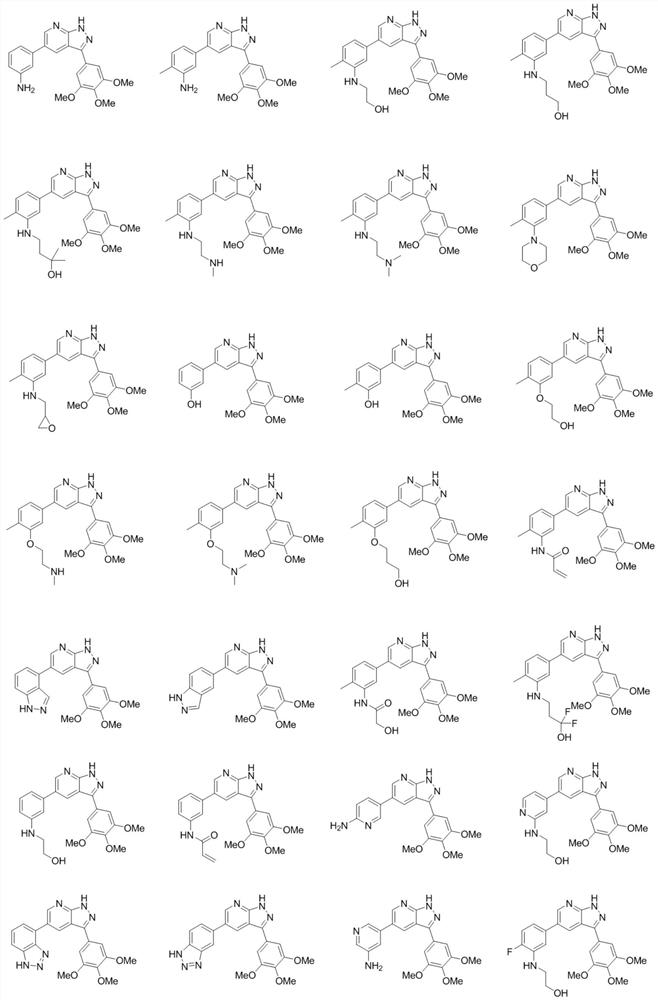
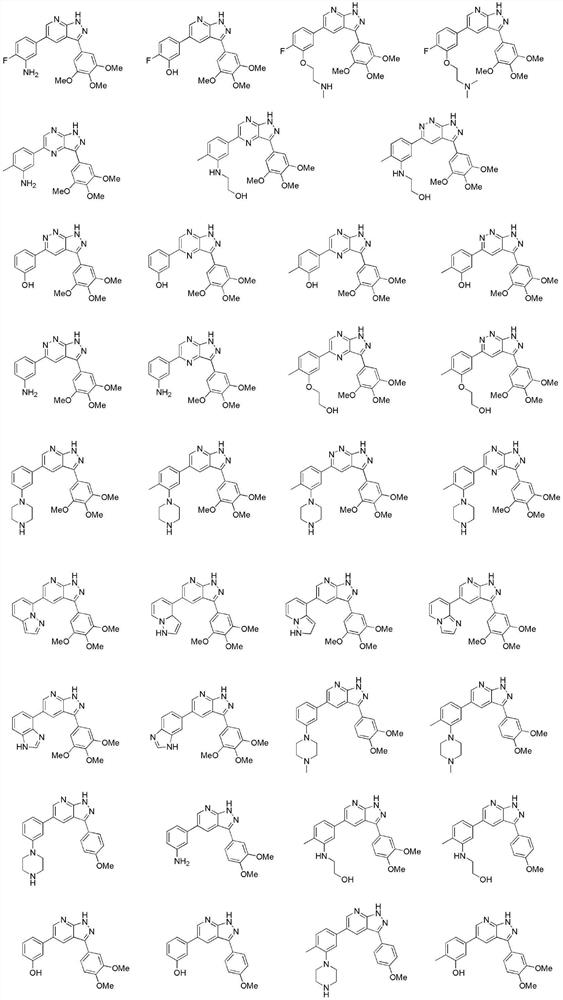
Image
Examples
preparation example Construction
[0096] The present invention also provides a method for preparing a nitrogen-containing heterocyclic compound in an embodiment, which is a method for preparing the nitrogen-containing heterocyclic compound in the above embodiment, comprising the following steps:
[0097] Step S110: compound A, R 1 B(OH) 2 , palladium catalyst and basic reagent reaction, preparation compound B.
[0098] Wherein, the structural formula of compound A is The structural formula of compound B is Y and Z are each independently selected from one of -CH and -N.
[0099] In one embodiment, the palladium catalyst is PdCl 2 (dppf) is (1,1'-bis(diphenylphosphino)ferrocene palladium chloride). The alkaline reagent is sodium carbonate.
[0100] Specifically, compound A, R 1 B(OH) 2 In the step of reacting the palladium catalyst and the alkaline reagent, a degassed aqueous solution of dioxane is also added as a solvent.
[0101] Further, in step S110, the reaction temperature is 80°C-100°C, and the r...
Embodiment 1
[0144] The structural formula of the nitrogen-containing heterocyclic compound of the present embodiment is: The preparation process is as follows:
[0145] (1)
[0146] Weigh compound 1 (2g, 6.19mmol) and dissolve it in DMF, cool in an ice bath to 0°C, add NaH (0.5g, 12.39mmol) slowly, stir for 10 minutes, add SEMCl (2mL, 10.53mmol) dropwise, and stir at room temperature reaction. After the reaction was completed, water was added to quench the reaction, extracted with ethyl acetate (EA), the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain 2 g of a light yellow solid with a yield of 71.4%, which was Compound 2 . The structural characterization of compound 2 is shown below: LC-MS: m / z 454[M+H] + . 1 H NMR (400MHz, CDCl 3 )δ8.62(d, J=2Hz, 1H), 7.99(d, J=2Hz, 1H), 5.83(S, 2H), 3.66(t, J=7.2Hz, 2H), 0.95(t, J= 7.2Hz,2H),-0.03(s,9H). 13 C NMR (100MHz, CDCl 3 )δ151.3, 149.5, 132.5, 122.1, 113...
Embodiment 2
[0154] The structural formula of the nitrogen-containing heterocyclic compound of the present embodiment is: The preparation process is as follows:
[0155] (1)
[0156] Compound 6 (100mg, 0.192mmol) was dissolved in DMF (3mL), and K 2 CO 3 (79mg, 0.576mmol), the temperature was raised to 80°C and compound 8 (72mg, 0.576mmol) was added in portions, and reacted overnight. After the reaction was completed, water was added to quench the reaction, EA was extracted, filtered, and the crude product obtained after concentration was compound 9, which was directly used in the next reaction. The structure of the crude product was characterized as follows: LC-MS: m / z 565 [M+H] + .
[0157] (2)
[0158] Compound 9 was dissolved in ethanol (6 mL), added with 2N HCl (6 mL), and reacted overnight at 80°C. After the reaction, it was concentrated under reduced pressure, separated and purified in liquid phase, and freeze-dried to obtain the yellow target compound 10, which is the ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com