Tablet coating film, raw material composition and preparation of tablet coating film, controlled release tablet and preparation of controlled release tablet
A raw material composition, coating film technology, applied in the directions of pill delivery, sugar-coated pills, drug delivery, etc., can solve problems such as the inability to achieve continuous production, and achieve constant drug release rate, high product safety, and low production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The raw material composition of the tablet coating film of the present embodiment is made of the material of following weight ratio:
[0071]
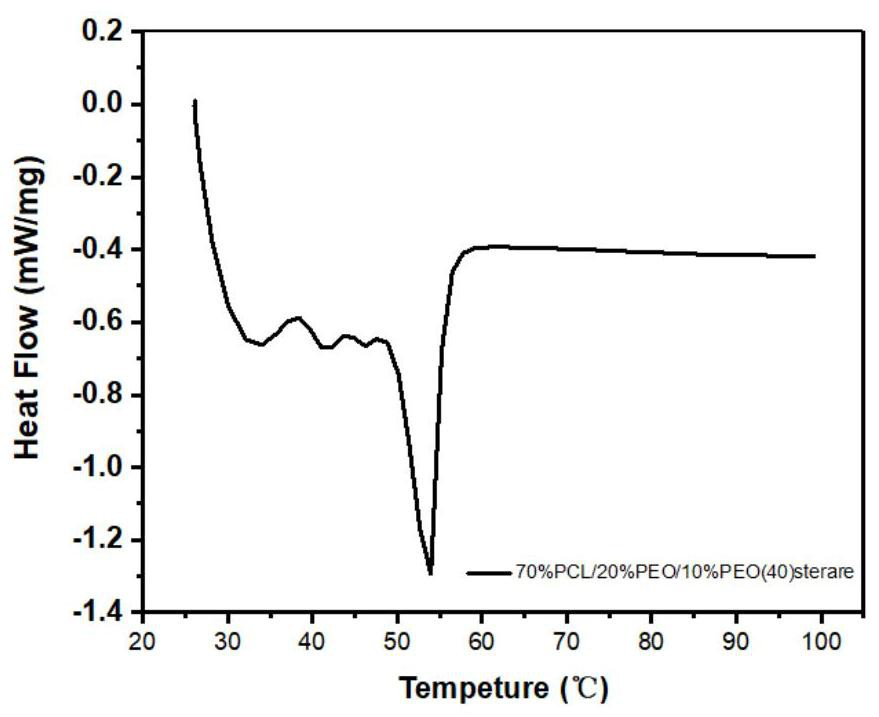
[0072] Preparation process: According to the above weight ratio, the ingredients in the raw material composition of the tablet coating film are dried for 24 hours, wherein polycaprolactone is the film-forming material, polyoxyethylene is the porogen, and polyoxyethylene (40) stearin The acid ester is a plasticizer, and the benzoyl peroxide is a crosslinking agent. At 120 DEG C, the film-forming material, pore-forming agent and plasticizer were hot-melt mixed for 5 minutes and then the temperature was lowered to 70 DEG C, adding benzoyl peroxide accounting for 2% by weight of the raw material composition of the tablet coating film as Crosslinker, continue mixing for 5 minutes.
[0073] Transfer the hot-melt mixture to the mold of a flat vulcanizer. The thickness of the mold is 0.2mm. It is pre-pressed three times by infrared ...
Embodiment 2
[0079] The raw material composition of the tablet coating film of the present embodiment is made of the material of following weight ratio:
[0080]
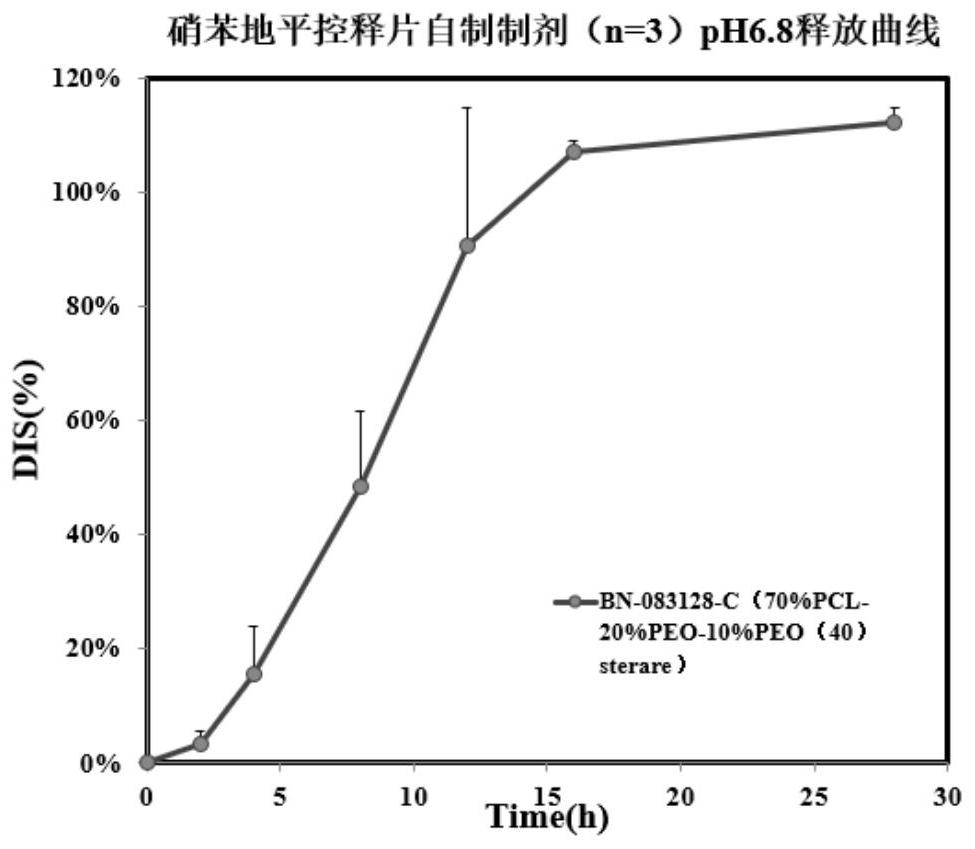
[0081] Preparation process: According to the above weight ratio, the ingredients in the raw material composition of the tablet coating film are taken and dried for 24 hours. Film-forming material (polycaprolactone), porogen (polyoxyethylene), plasticizer (polyoxyethylene (40) stearate) and crosslinking agent (accounting for the raw material composition of tablet coating film 2% by weight of benzoyl peroxide) is mixed and extruded by a hot-melt extruder, wherein the mixing temperature is 70°C, and the mixing time is 10min.
[0082]Transfer the hot-melt mixture to a mold with a thickness of 0.2mm, pre-press three times by heating to 70°C and 0MPa by infrared or microwave to remove the air in the mold, hot-press at 120°C and 3MPa for 5 minutes, take out the mold, and unload Drop the pressure, cold press at 3MPa for 5 minutes, a...
Embodiment 3
[0087] The raw material composition of the tablet coating film of the present embodiment is made of the material of following weight ratio:
[0088]
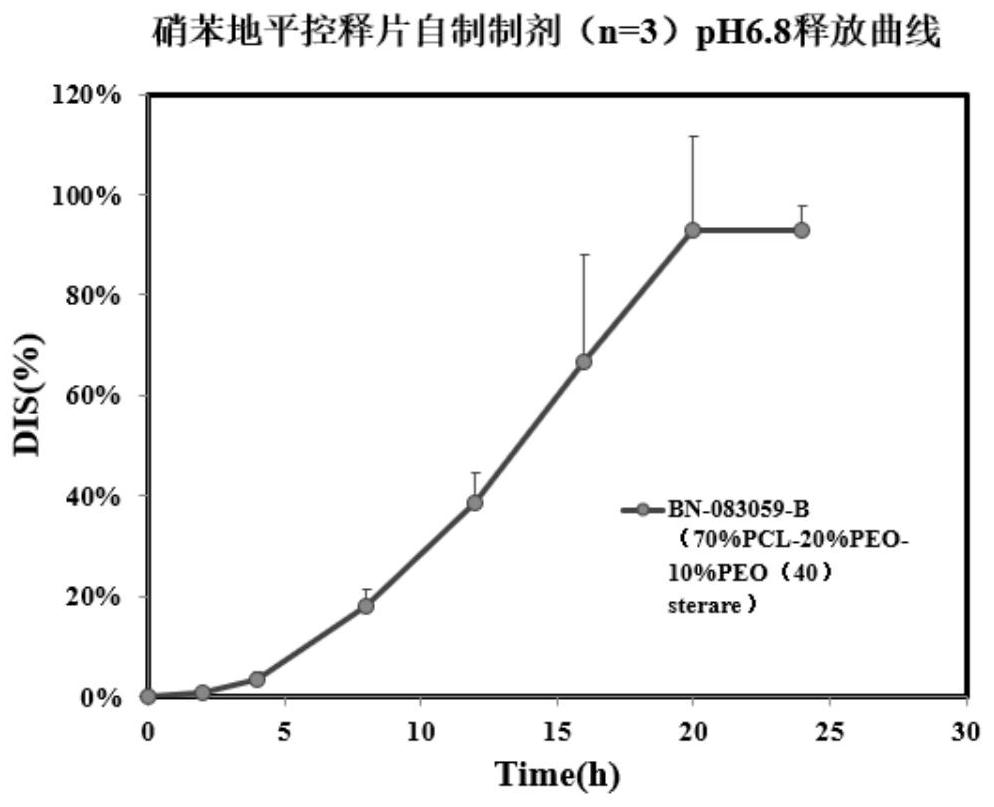
[0089] The preparation process of the controlled-release tablet of this embodiment is the same as that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com