Protein freeze-dried powder and solution thereof
A technology of freeze-dried powder and protein, applied in freeze-dried delivery, peptide/protein composition, powder delivery, etc., can solve the problems of unstable substance changes, drug failure, side effects, etc., to ensure stability, improve storage environment, and improve biological The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the prescription research of protein freeze-dried powder
[0041] 1. Prescription screening
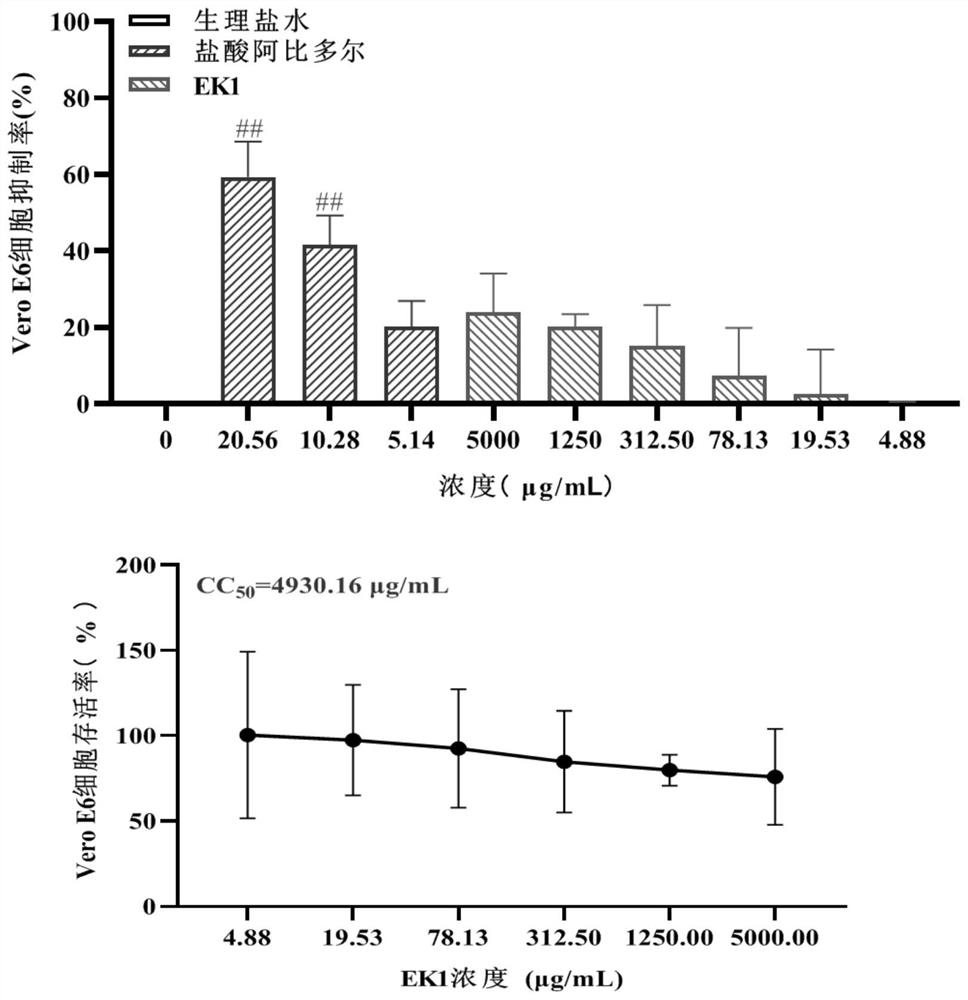
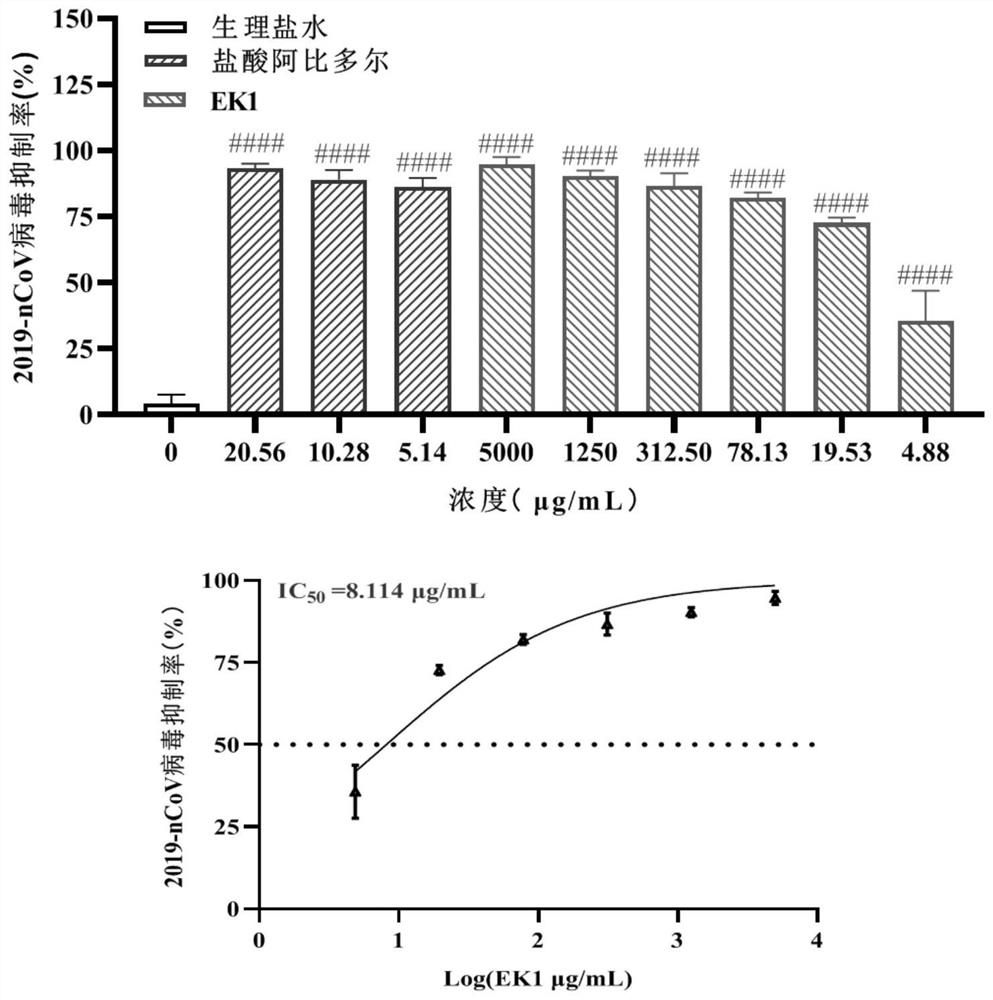
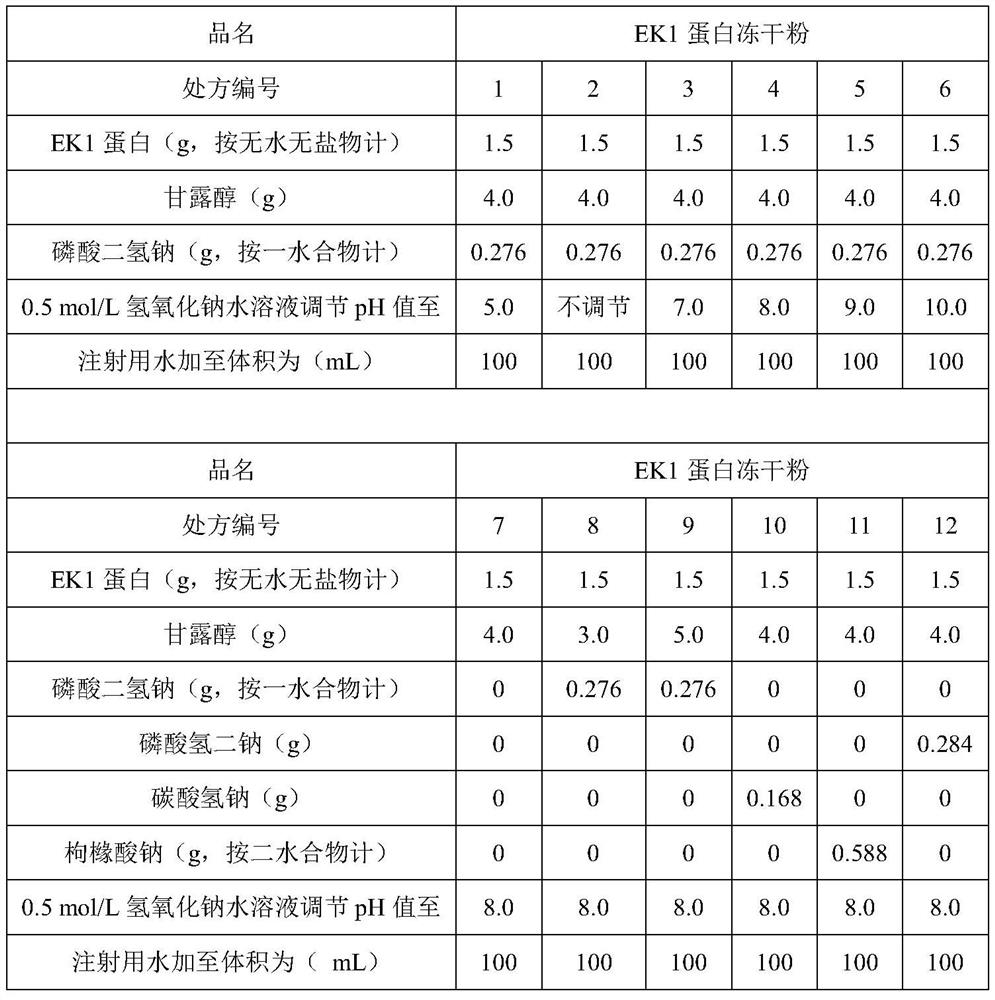
[0042] Taking EK1 protein as an example, its alkalinity (1mg / mL) is about 8.5, and considering that the theoretical isoelectric point of the protein is about 4.02, the conventional pH range of the combined preparation is 4-9, and the design pH range is 3-10 , the adjusted value is 3.0-10.0 respectively; buffer salt is added in the prescription to increase the pH stability of the preparation, buffer salt selection: sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium bicarbonate, sodium citrate; salt concentration selection: 20mmol / L; the pH regulator is 0.5mol / L hydrochloric acid solution or 0.5mol / L sodium hydroxide solution; appropriate amount of mannitol is added as excipient in the prescription, and the prescription is designed.
[0043] Weigh the prescribed amount of mannitol, sodium dihydrogen phosphate and EK1 protein, add an appropriate amoun...
Embodiment 2
[0089] Embodiment 2: Research on the preparation process of protein freeze-dried powder
[0090] 1. Freeze-drying process
[0091] The eutectic point of the EK1 protein prescription solution is -15°C to -25°C.
[0092] At room temperature and normal pressure, place the EK1 protein prescription solution in a lyophilizer, cool down to -50°C, pre-freeze for 1 hour, and keep for 5-6h; at 0.2-0.4mbar, heat up to -20°C, and keep for 18-24h ; Under the condition of 0-0.2mbar, raise the temperature to 0°C; raise the temperature to 25°C, keep it for 5h, then the freeze-drying can be completed, and the protein freeze-dried powder can be obtained.
[0093] 2. Selection of filter material
[0094] Under normal temperature conditions, to simulate the maximum filtration capacity in the actual filtration production of drugs, according to the adsorption capacity of the hydrophilic polyethersulfone (PES) membrane and hydrophilic polyvinylidene fluoride (PVDF) membrane to the product EK1 prot...
Embodiment 3
[0097] Embodiment 3: investigation of atomization stability
[0098] Atomization device 1: Compression atomizer liquid device (Omron NE-C25S type)
[0099] Test one:
[0100] Test design:
[0101] Compression nebulizer liquid device manufacturer: Omron, model: NE-C25S; liquid medicine filling volume of the liquid medicine cup: 2 ~ 6mL.
[0102] Take 2 bottles of 3 batches of protein freeze-dried powder (prescription 4) (batch numbers: 200501, 200502, 200601), add 3mL of 0.9% sodium chloride injection to dissolve each bottle, shake well, and transfer the solution of 2 bottles to the compression type Mix well in the medicine solution cup of the nebulizer, and the medicine solution cup assembly is sealed and connected to the dropper; add 5mL of water to the 15mL vial as the receiving solution, place the mouth of the dropper under the liquid surface, turn on the nebulizer for atomization sampling, and take 20 minutes Sampling once, sampling twice (the overall atomization time i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com