Method for preparing human basic fibroblast growth factor by using bacillus subtilis and endonuclease
A nucleic acid construct and intein technology, applied in the field of biology, can solve the problem that the factors of intein cleavage are not very clear and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Expression vector construction and host cell transformation
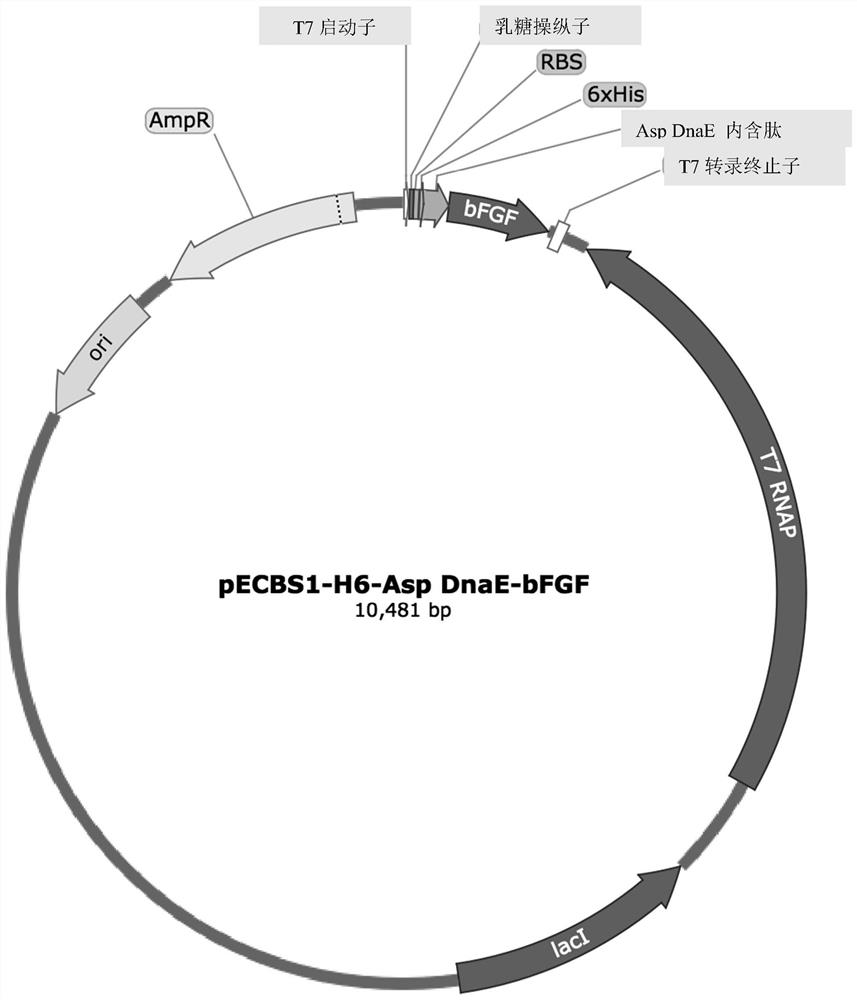
[0093] Construction and Design of Escherichia coli / Bacillus subtilis Expression Shuttle Vector
[0094] pRB374 and pBR322 were used as the starting vector of E. coli / Bacillus subtilis expression shuttle vector [14]. Specifically, pECBS1 was constructed by the following modification steps: first, pRB374 (5.9 kb) was digested with SalI and BglII; T7 ribonucleic acid polymerase-Lac promoter-LacI gene-LacI q The promoter-bleomycin resistance gene-partial neomycin resistance gene fragment (5.3kb) was substituted to form pECBSi vector. Then, the formed pECBSi vector and pBR322 vector were digested with EcoRI and BglI, respectively, and the pECBSi digested fragment was replaced with the fragment obtained by digesting pBR322 (4.3 kb), thereby forming the pECBS1 shuttle vector.
[0095] Construction of bFGF expression vector
[0096] The construction method of Escherichia coli / Bacillus subtilis expr...
Embodiment 2
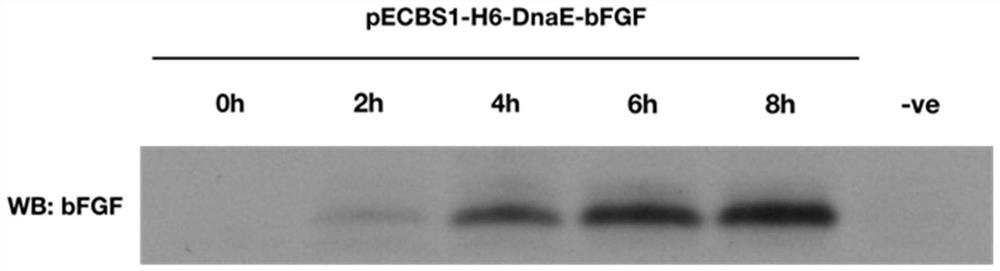
[0099] Example 2: Expression of bFGF
[0100] shake flask culture
[0101] B. subtilis transformants were grown at 37°C (250 rpm) in 200 ml 2x LB medium supplemented with 25 μg / ml kanamycin [15]. When the A600 value reached 1.0, IPTG was added at a final concentration of 0.2 mM, and then 1 ml of culture samples were collected at 3-hour intervals for bFGF expression analysis. The cell pellet was resuspended in 200 μl of resuspension buffer (50 mM Tris-Cl, 200 mM EDTA, pH 8.0) and incubated on ice for 5 minutes. The mixture was then treated with 120 μl of lysozyme solution (10 mg / mL) for 20 minutes at 37°C. Then 80 μl of lysis buffer (10 mM EDTA, 10% Triton X-100 and 50 mM Tris-Cl, pH 8.0) was added. The tube containing the solution was gently inverted and then centrifuged at 14,800 rpm for 5 minutes. Cell lysate samples were analyzed by Western blot for bFGF protein expression.
[0102] In order to successfully express the soluble bFGF protein, the inventors also tested ...
Embodiment 3
[0109] Example 3: Purification and structural determination of bFGF protein
[0110] Cation exchange chromatography and heparin-agarose chromatography were used to purify bFGF. First, the protein concentration of the eluted fractions was measured using a Nanodrop Microvolume spectrophotometer. In addition, eluted fractions with significant readings (approximately 1 mg / ml) were pooled and dialyzed against 0.1x PB. Afterwards, the purified bFGF band was obtained by electrophoresis on a 10% SDS-PAGE gel stained with Coomassie brilliant blue R-250. The band containing bFGF protein in the SDS-PAGE gel was recovered for subsequent analysis by LC-MS.
[0111] The results of Western blot analysis showed that the soluble bFGF protein extracted from the lysate had the same molecular weight as the bFGF protein purchased from Thermofisher Scientific ( FIG. 4 ). Purified bFGF protein samples were subjected to N-terminal and C-terminal protein sequencing and MALDI-TOF mass determination ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com