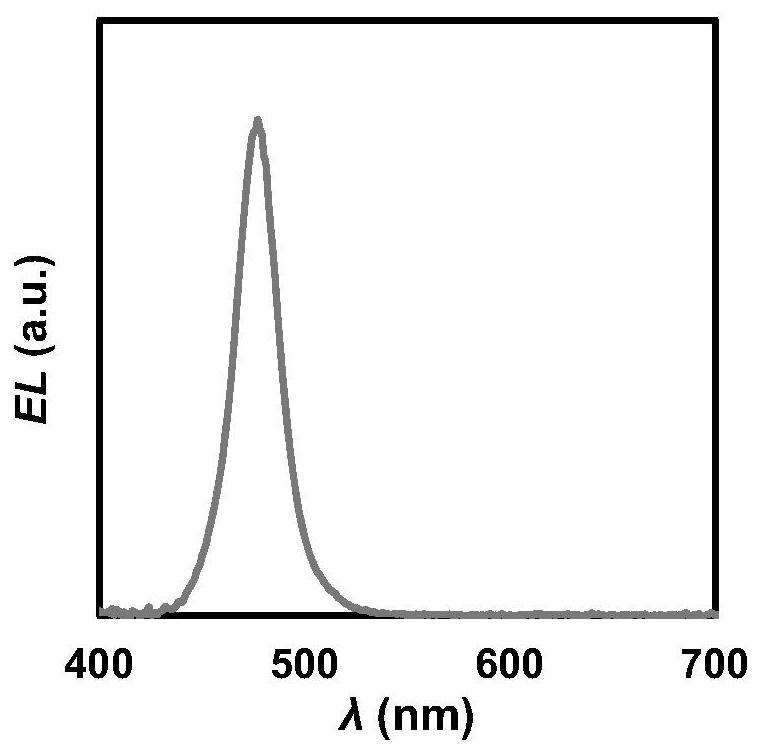
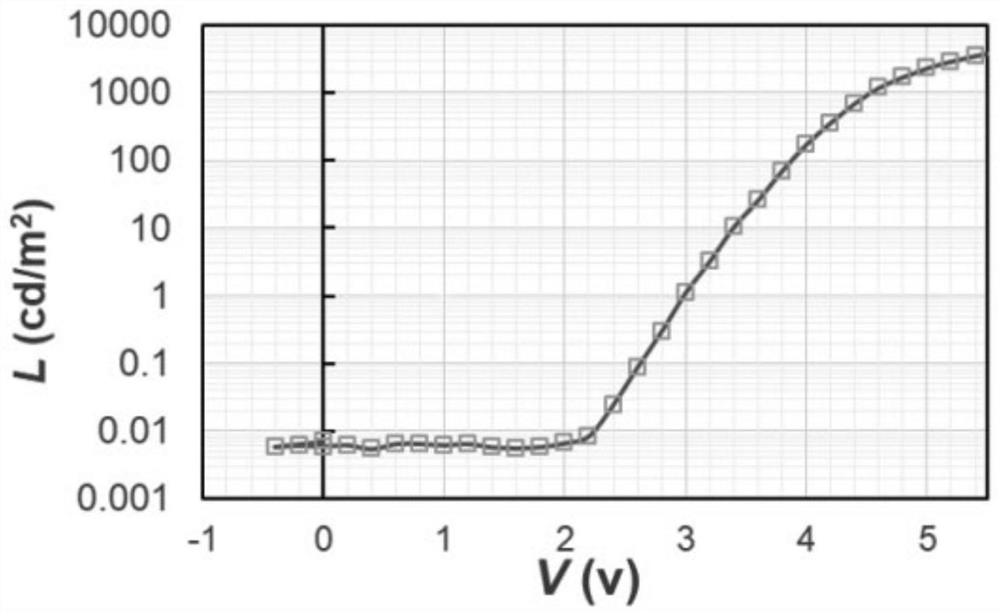
Triarylamine polymer, preparation method and application thereof
A technology of polymer and triarylamine, which is applied in the field of triarylamine polymer and its preparation, can solve the problems of limited use, low glass transition temperature, hole mobility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] Embodiment 1——Preparation of 3-trifluoromethoxy polytriphenylamine
[0214] 1. Preparation of Monomer 3-Trifluoromethoxytriphenylamine
[0215] In an open round bottom flask equipped with a magnetic stirrer and a thermometer, add 5 g of diphenylamine, 12.8 g of m-iodotrifluoromethoxybenzene, 1.5 mmol of palladium acetate, 6.2 g of potassium carbonate, and 250 mL of dimethyl sulfoxide (DMSO ) solvent, oscillating and mixing evenly and then warming up to 120°C and stirring the reaction under air, monitoring the reaction progress with TLC, stopping heating after the reaction is complete, quenching the reaction with saturated ammonium chloride solution after the mixture in the round bottom flask is cooled, and reacting After the mixture was extracted, dried, and concentrated under reduced pressure, the crude product obtained was separated and purified by column chromatography, and the eluent used was petroleum ether. The column chromatography eluate containing the product...
Embodiment 2
[0231] Embodiment 2——Preparation of 3-trifluoromethyl polytriphenylamine
[0232] 1. Preparation of Monomer 3-Trifluoromethyltriphenylamine
[0233] In an open round bottom flask equipped with a magnetic stirrer and a thermometer, add 4.96g of diphenylamine, 11.98g of m-iodobenzobenzotrifluoride, 1.5mmol of palladium acetate, 6.1g of potassium carbonate and 200mL of dimethylsulfoxide (DMSO) solvent , oscillate and mix evenly, heat up to 120°C and stir the reaction under air, monitor the reaction progress with TLC, stop heating after the reaction is complete, quench the reaction with saturated ammonium chloride solution after the mixture in the round bottom flask is cooled, and the reaction mixture The crude product obtained after extraction, drying, and concentration under reduced pressure is separated and purified by column chromatography, and the eluent used is petroleum ether. The column chromatography eluate containing the product is collected and concentrated to obtain ...
Embodiment 3
[0248] Embodiment 3——Preparation of 4-trifluoromethoxy polytriphenylamine
[0249] 1. Preparation of monomer 4-trifluoromethoxytriphenylamine
[0250] In an open round bottom flask equipped with a magnetic stirrer and a thermometer, add 5.1 g of diphenylamine, 13.06 g of p-bromotrifluoromethoxybenzene, 1.5 mmol of palladium acetate, 6.46 g of potassium carbonate and 200 mL of dimethyl sulfoxide ( DMSO) solvent, oscillating and mixing evenly, then warming up to 125°C and stirring the reaction under air, monitoring the progress of the reaction with TLC, stopping the heating after the reaction is complete, and quenching the reaction with saturated ammonium chloride solution after the mixture in the round bottom flask is cooled. The crude product obtained after the reaction mixture was extracted, dried, and concentrated under reduced pressure was separated and purified by column chromatography, and the eluent used was petroleum ether. The column chromatography eluate containing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hole mobility | aaaaa | aaaaa |
| Hole mobility | aaaaa | aaaaa |
| Hole mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com