Monoclonal antibody against novel coronaviruses and application of monoclonal antibody
A monoclonal antibody and anti-idiotypic antibody technology, which is applied in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problem that there is no specific drug for the new coronavirus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: Expression and purification of 2019-nCoV virus S protein RBD
[0094] Using NdeI and XhoI enzymes, the DNA fragment encoding the 2019-nCoV / 2019 strain spike protein S protein RBD (its amino acid sequence is shown in SEQ ID NO: 17) was connected to the pET21a vector, and the DNA fragment was in the coding region A nucleotide sequence encoding a 6*histidine tag (6*His tag) and a stop codon are also connected to the 3' end of the tag. The ligation product was transformed into BL21 E. coli competent cells. Then, pick a single clone, inoculate into 40mL LB medium, and cultivate for 6-8 hours; then inoculate into 4L of LB medium, and cultivate to OD600=0.4-0.6 at 37 degrees Celsius. Subsequently, IPTG was added to the culture to a final concentration of 1 mM, and the culture was continued at 37 degrees Celsius for 4-6 hours. After culturing, the inclusion bodies were harvested and refolded. The refolded protein solution was concentrated and dialyzed into 20mM Tr...
Embodiment 2
[0095] Example 2: Isolation of memory B cells that specifically recognize RBD proteins
[0096] With the informed consent of those infected with 2019-nCoV who were cured and discharged, 10 mL of blood was collected to separate PBMCs. Separated PBMCs in 10 7 / mL density and final concentration of 400nM RBD protein (prepared as in Example 1) were incubated on ice for half an hour; then washed 2 times with PBS, and then incubated with the following antibodies (both purchased from BD): anti-human CD3 / PE-Cy5, anti-human CD16 / PE-Cy5, anti-human CD235a / PE-Cy5, anti-human CD19 / APC-Cy7, anti-human CD27 / Pacific Blue, anti-human CD38 / APC, anti- human IgG / FITC, and anti-His / PE. After incubation on ice for half an hour, wash PBMCs twice with PBS. Subsequently, sort PBMCs with FACSAria III and collect PE - Cy5 - APCs - APC-Cy7 + Pacific Blue + FITC + PE + The cells (i.e. B cells) were directly collected into a 96-well plate, 1 cell / well.
Embodiment 3
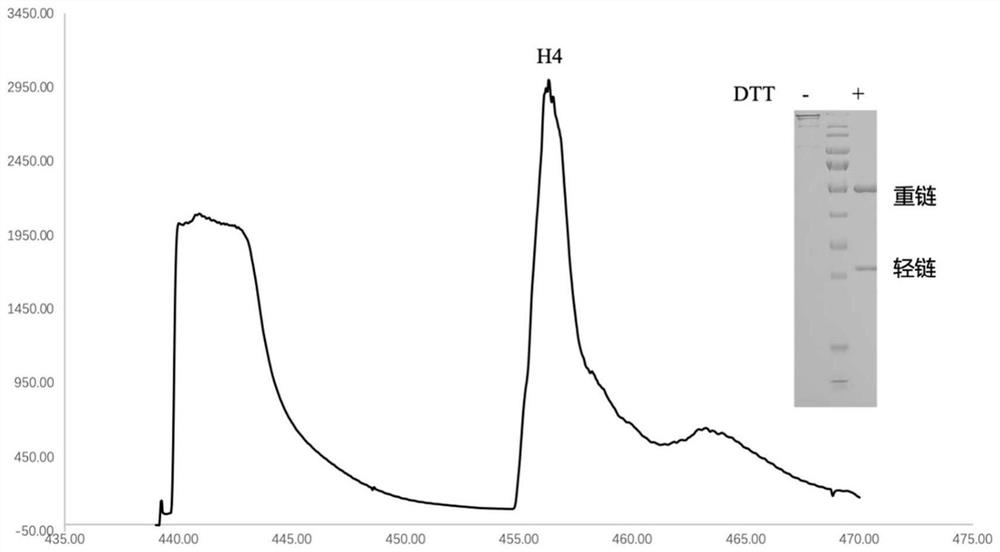
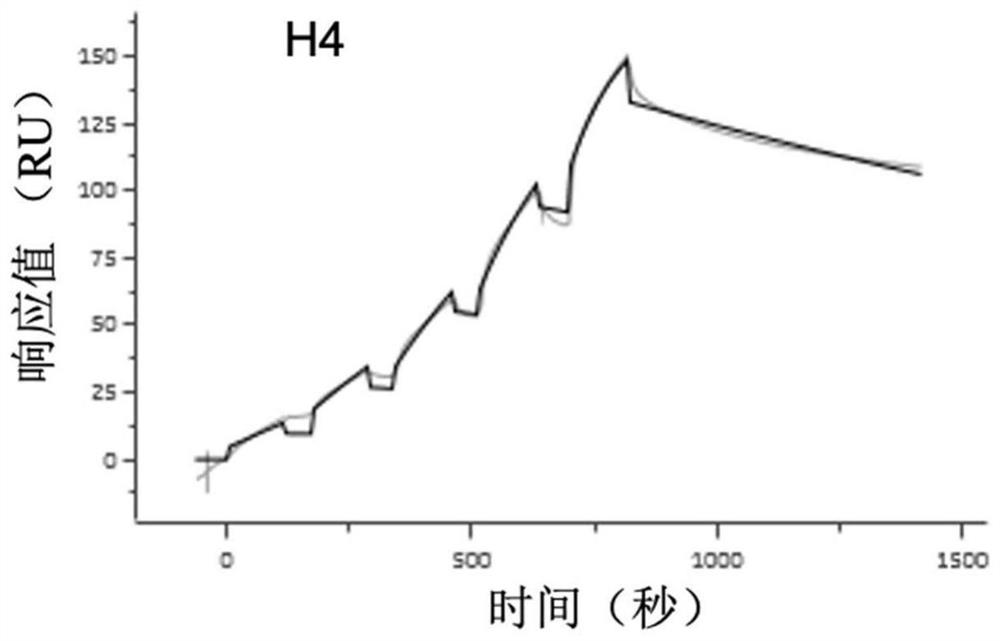
[0097] Example 3: Isolation and identification of H4 antibody and construction of recombinant expression vector
[0098] The B cells obtained in Example 2 were reverse-transcribed using Superscript III reverse transcriptase (Invitrogen) (at 55°C for 60 minutes), wherein the reverse transcription primers used are shown in Table 2.
[0099] Table 2. Sequence information of the reverse transcription primers used
[0100]
[0101] Using the reverse transcription product as a template, the first round of PCR (PCRa) was carried out with HotStar Tap Plus enzyme (QIAgen) to amplify the sequence of the variable region of the antibody; wherein, the primers used are shown in Table 3; the reaction conditions used As follows: 95°C, 5min; 35 cycles of (95°C for 30s, 55°C (heavy chain / κ chain) for 30s, 72°C for 90s); 72°C, 7min. Subsequently, the second round of PCR (PCRb) was carried out using the amplified product as a template; wherein, the primers used were as shown in Table 4; the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com