A kind of preparation method and application of 3-phenyl-2-propen-1-one o-n-butyl oxime
A technology of n-butyl oxime and phenyl, which is applied in the field of organic synthesis, can solve the problems of strong toxicity and unsuitability for large-scale production, and achieve the effects of convenient operation, low energy consumption, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
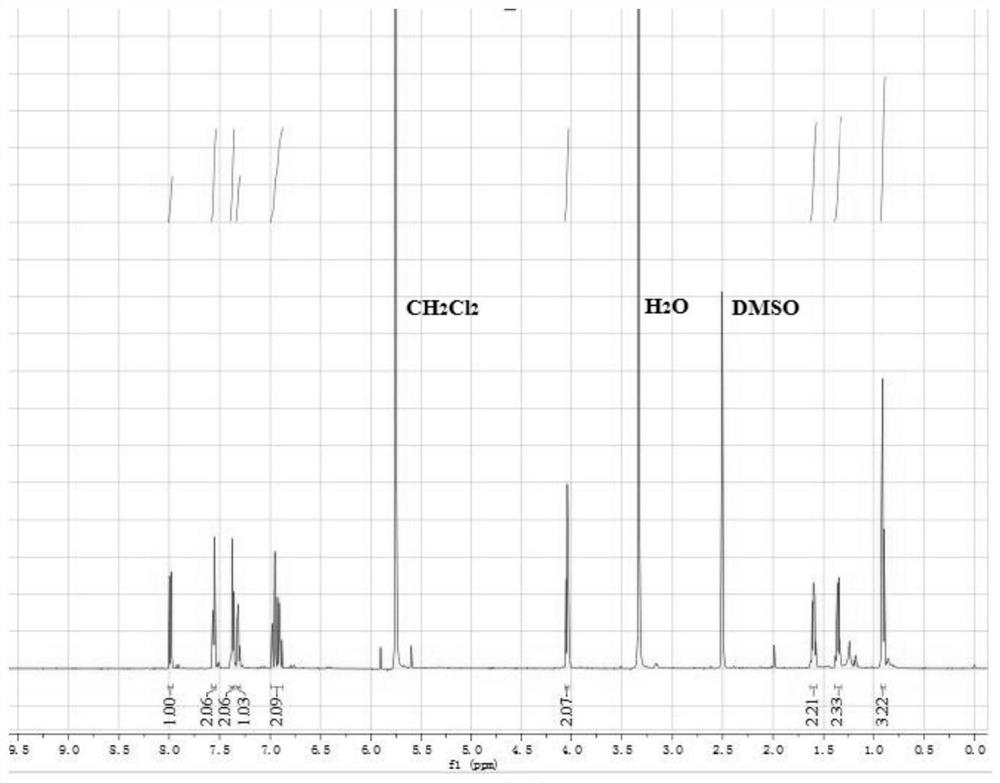
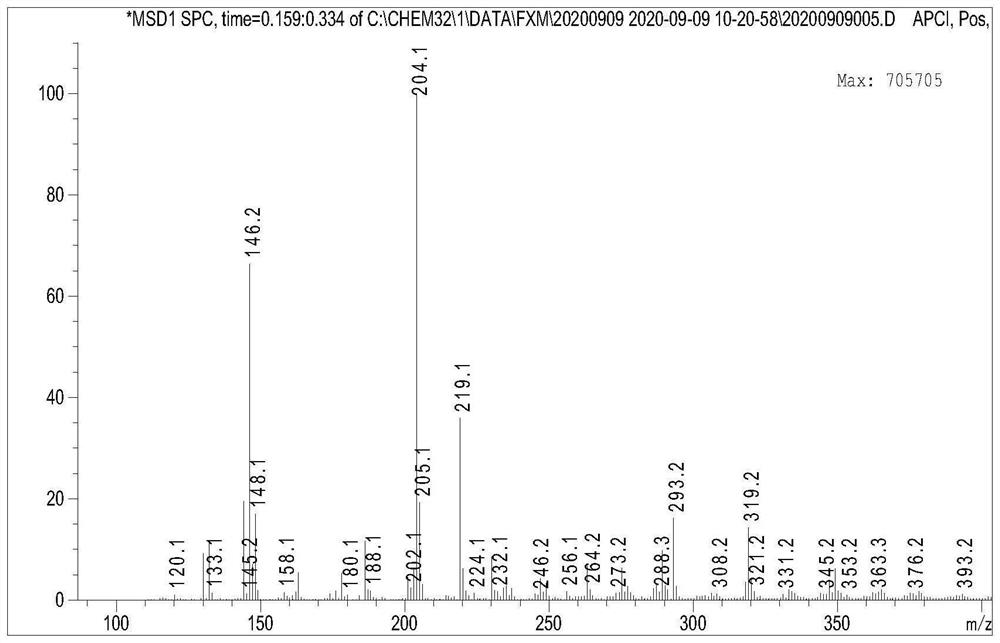
[0037] A magnetic stirring bar was added to a 100mL single-necked flask, and 1.32g (0.01mol) of cinnamaldehyde, 0.86g (0.0125mol) of hydroxylamine hydrochloride, 1.37g (0.01mol) of bromobutane, and 5g of potassium hydroxide were added to the round-bottomed flask, Add 2 mL of ethanol, H 2 O5mL, the temperature was raised to 80°C and the reaction was stirred for 5 hours. After the completion of the reaction, the reaction was carried out under reduced pressure for precipitation, and flash column chromatography was performed using petroleum ether and ethyl acetate (50:1) to obtain 3-phenyl-2-propen-1-one O-n-butyl oxime, which was pale Yellow oily liquid (yield: 78%). The NMR results of the compounds are figure 1 , figure 2 shown.
[0038] compound 1 H-NMR (600MHz, DMSO) δ: 7.99 (d, J=9.3 Hz, 1H), 7.56 (d, J=7.5 Hz, 2H), 7.38 (t, J=7.5 Hz, 2H), 7.32 (t, J=7.3Hz, 1H), 6.93(dt, J=16.0, 12.7Hz, 2H), 4.04(t, J=6.6Hz, 2H), 1.64(m, 2H), 1.40(m, 2H), 0.91( t, J=7.4 Hz, 3H), M / z: ...
Embodiment 2
[0040] A magnetic stirring bar was added to the 100mL single-necked flask, and 1.32g (0.01mol) of cinnamaldehyde, 0.86g (0.0125mol) of hydroxylamine hydrochloride, 1.51g (0.011mol) of bromo-n-butane, and 5g of potassium hydroxide were added to the round-bottomed flask, Add 2 mL of ethanol, H 2O 5mL, the temperature was raised to 80°C and the reaction was stirred for 5 hours. After the completion of the reaction, it was desolvated under reduced pressure, and flash column chromatography was performed using petroleum ether and ethyl acetate (50:1) to obtain 3-phenyl-2-propen-1-one O-n-butyl oxime, which was pale Yellow oily liquid (yield: 82%).
Embodiment 3
[0042] A magnetic stirring bar was added to a 100mL single-necked flask, and 1.32g (0.01mol) of cinnamaldehyde, 0.86g (0.0125mol) of hydroxylamine hydrochloride, 1.71g (0.0125mol) of bromo-n-butane, and 5g of potassium hydroxide were added to the round-bottomed flask, Add 2 mL of ethanol, H 2 O 5mL, the temperature was raised to 80°C and the reaction was stirred for 5 hours. After the completion of the reaction, it was desolvated under reduced pressure, and flash column chromatography was performed using petroleum ether and ethyl acetate (50:1) to obtain 3-phenyl-2-propen-1-one O-n-butyl oxime, which was pale Yellow oily liquid (yield: 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com